1. Balzer I, Hardeland R. Melatonin in algae and higher plantsŌĆÉpossible new roles as a phytohormone and antioxidant. Bot Acta 1996;109:180ŌĆō183.

2. Vivien-Roels B, P├®vet P. Melatonin: presence and formation in invertebrates. Experientia 1993;49:642ŌĆō647.

3. Vivien-Roels B, Pevet P, Beck O, Fevre-Montange M. Identification of melatonin in the compound eyes of an insect, the locust (Locusta migratoria), by radioimmunoassay and gas chromatography-mass spectrometry. Neurosci Lett 1984;49:153ŌĆō157.


4. Anisimov VN, Mylnikov SV, Oparina TI, Khavinson VK. Effect of melatonin and pineal peptide preparation epithalamin on life span and free radical oxidation in Drosophila melanogaster. Mech Ageing Dev 1997;97:81ŌĆō91.


5. Antol├Łn I, Obst B, Burkhardt S, Hardeland R. Antioxidative protection in a high-melatonin organism: the dinoflagellate Gonyaulax polyedra is rescued from lethal oxidative stress by strongly elevated, but physiologically possible concentrations of melatonin. J Pineal Res 1997;23:182ŌĆō190.


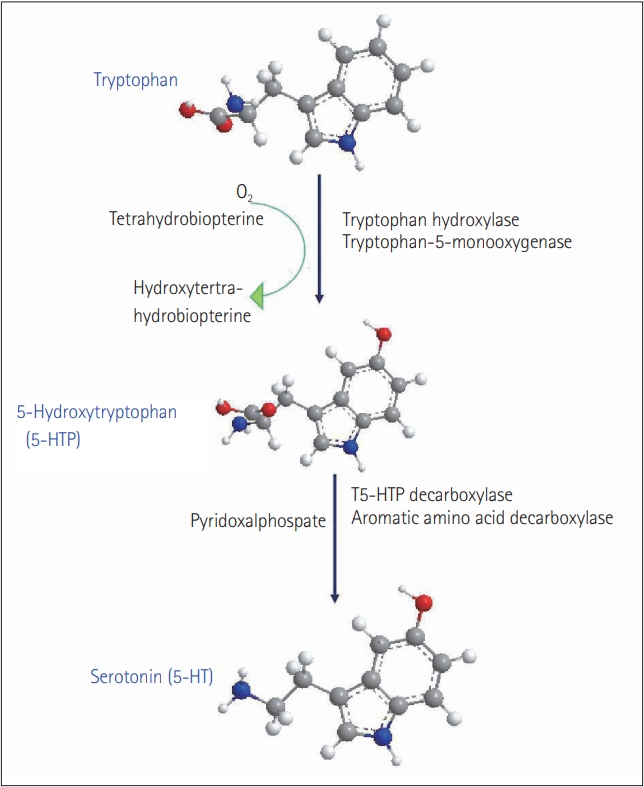
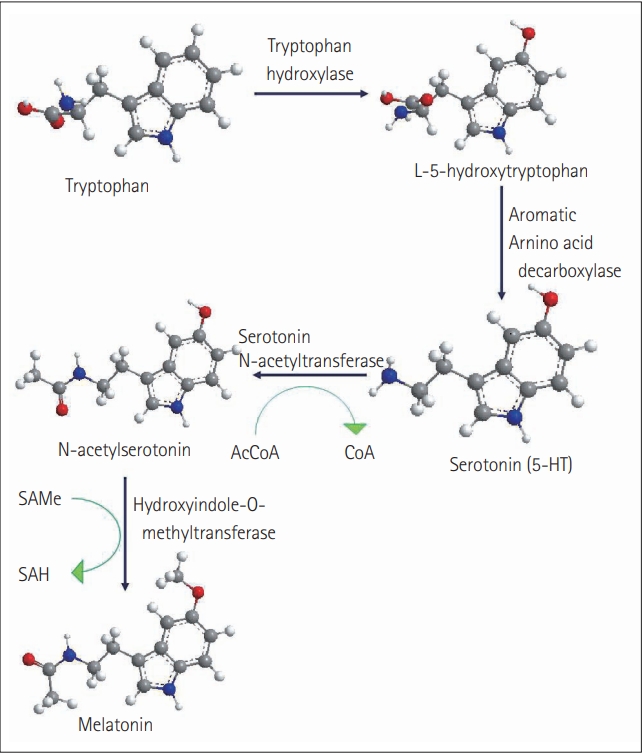
7. Fitzpatrick PF. Tetrahydropterin-dependent amino acid hydroxylases. Annu Rev Biochem 1999;68:355ŌĆō381.


9. Kim-Ha J, Smith JL, Macdonald PM. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell 1991;66:23ŌĆō35.


10. Lovenberg W, Weissbach H, Udenfriend S. Aromatic L-amino acid decarboxylase. J Biol Chem 1962;237:89ŌĆō93.


11. Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr Rev 1991;12:151ŌĆō180.


12. Arendt J. Melatonin and the mammalian pineal gland. London: Chapman & Hall, 1995.
13. Klein DC, Weller JL. Indole metabolism in the pineal gland: a circadian rhythm in N-acetyltransferase. Science 1970;169:1093ŌĆō1095.


14. Thomas KB, Iuvone PM. Circadian rhythm of tryptophan hydroxylase activity in chicken retina. Cell Mol Neurobiol 1991;11:511ŌĆō527.


15. Evans PH, Fox PM. Enzymatic N-acetylation of indolealkylamines by brain homogenates of the honeybee, Apis mellifera. J Insect Physiol 1975;21:343ŌĆō353.

16. Smith TJ. Phylogenetic distribution and function of arylalkylamine N-acetyltransferase. Bioessays 1990;12:30ŌĆō33.


17. Takeda M, Endo Y, Saito H, Nishimura M, Nishitsutsuji-Uwo J. Neuropeptide and monoamine immunoreactivity of the circadian pacemaker in Periplaneta. Biomed Res 1985;6:395ŌĆō406.

18. Rapport MM, Green AA, Page IH. Serum vasoconstrictor, serotonin; isolation and characterization. J Biol Chem 1948;176:1243ŌĆō1251.

19. Rapport MM, Green AA, Page IH. Crystalline serotonin. Science 1948;108:329ŌĆō330.


20. Neckameyer WS. A trophic role for serotonin in the development of a simple feeding circuit. Dev Neurosci 2010;32:217ŌĆō237.


21. Dacks AM, Nickel T, Mitchell BK. An examination of serotonin and feeding in the flesh fly Neobellieria bullata (Sarcophagidae: Diptera). J Insect Behav 2003;16:1ŌĆō21.
23. Novak MG, Ribeiro JM, Hildebrand JG. 5-hydroxytryptamine in the salivary glands of adult female Aedes aegypti and its role in regulation of salivation. J Exp Biol 1995;198:167ŌĆō174.


24. Novak MG, Rowley WA. Serotonin depletion affects blood-feeding but not host-seeking ability in Aedes triseriatus (Diptera: Culicidae). J Med Entomol 1994;31:600ŌĆō606.


25. Berridge MJ, Patel NG. Insect salivary glands: stimulation of fluid secretion by 5-hydroxytryptamine and adenosine-3ŌĆÖ,5ŌĆÖ-monophosphate. Science 1968;162:462ŌĆō463.


26. Donini A, OŌĆÖDonnell MJ, Orchard I. Differential actions of diuretic factors on the Malpighian tubules of Rhodnius prolixus. J Exp Biol 2008;211:42ŌĆō48.


27. Maddrell SH, Herman WS, Mooney RL, Overton JA. 5-Hydroxytryptamine: a second diuretic hormone in Rhodnius prolixus. J Exp Biol 1991;156:557ŌĆō566.


28. Sitaraman D, LaFerriere H, Birman S, Zars T. Serotonin is critical for rewarded olfactory short-term memory in Drosophila. J Neurogenet 2012;26:238ŌĆō244.


30. Bicker G, Menzel R. Chemical codes for the control of behaviour in arthropods. Nature 1989;337:33ŌĆō39.


31. Menzel R, Heyne A, Kinzel C, Gerber B, Fiala A. Pharmacological dissociation between the reinforcing, sensitizing, and response-releasing functions of reward in honeybee classical conditioning. Behav Neurosci 1999;113:744ŌĆō754.


32. Mercer AR, Menzel R. The effects of biogenic amines on conditioned and unconditioned responses to olfactory stimuli in the honeybeeApis mellifera. J Comp Physiol 1982;145:363ŌĆō368.

33. Anstey ML, Rogers SM, Ott SR, Burrows M, Simpson SJ. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science 2009;323:627ŌĆō630.


34. Ott SR, Verlinden H, Rogers SM, Brighton CH, Quah PS, Vleugels RK, et al. Critical role for protein kinase A in the acquisition of gregarious behavior in the desert locust. Proc Natl Acad Sci U S A 2012;109:E381ŌĆōE387.


35. Dierick HA, Greenspan RJ. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat Genet 2007;39:678ŌĆō682.


37. Brookhart GL, Edgecomb RS, Murdock LL. Amphetamine and reserpine deplete brain biogenic amines and alter blow fly feeding behavior. J Neurochem 1987;48:1307ŌĆō1315.


38. Novak MG, Rowley WA. Serotonin depletion affects blood-feeding but not host-seeking ability in Aedes triseriatus (Diptera: Culicidae). J Med Entomol 1994;31:600ŌĆō606.


39. Dacks AM, Nickel T, Mitchell BK. An examination of serotonin and feeding in the flesh fly Neobellieria bullata (Sarcophagidae: Diptera). J Insect Behav 2003;16:1ŌĆō21.
40. Haselton AT, Downer KE, Zylstra J, Stoffolano JG. Serotonin inhibits protein feeding in the blow fly, Phormia regina (Meigen). J Insect Behav 2009;22:452ŌĆō463.

41. Wetterberg L, Hayes DK, Halberg F. Circadian rhythm of melatonin in the brain of the face fly, Musca autumnalis De Geer. Chronobiologia 1987;14:377ŌĆō381.

42. Binkley SA. The clockwork sparrow: time, clocks, and calendars in biological organisms. Englewood Cliffs: Prentice Hall, 1990.
43. Finocchiaro L, Callebert J, Launay JM, Jallon JM. Melatonin biosynthesis in Drosophila: its nature and its effects. J Neurochem 1988;50:382ŌĆō387.


44. Itoh MT, Hattori A, Nomura T, Sumi Y, Suzuki T. Melatonin and arylalkylamine N-acetyltransferase activity in the silkworm, Bombyx mori. Mol Cell Endocrinol 1995;115:59ŌĆō64.


45. Tilden AR, Anderson WJ, Hutchison VH. Melatonin in two species of damselfly, Ischnura verticalis and Enallagma civile. J Insect Physiol 1994;40:775ŌĆō780.

46. Gorbet DJ, Steel CG. A miniature radioimmunoassay for melatonin for use with small samples from invertebrates. Gen Comp Endocrinol 2003;134:193ŌĆō197.


47. Vieira R, M├Łguez JM, Aldegunde M. GABA modulates day-night variation in melatonin levels in the cerebral ganglia of the damselfly Ischnura graellsii and the grasshopper Oedipoda caerulescens. Neurosci Lett 2005;376:111ŌĆō115.


48. Yang L, Qin Y, Li X, Song D, Qi M. Brain melatonin content and polyethism in adult workers of Apis mellifera and Apis cerana (Hym., Apidae). J Appl Entomol 2007;131:734ŌĆō739.

49. Itoh MT, Hattori A, Sumi Y, Suzuki T. Identification of melatonin in different organs of the cricket, Gryllus bimaculatus. Zool Sci 1994;11:577ŌĆō581.
50. Hodkova M. Indication of the role of melatonin in the regulation of reproduction in Pyrrhocoris apterus (Heteroptera). Acta Entomol Bohemos 1989;86:81ŌĆō85.
51. Hardie J, Gao N. Melatonin and the pea aphid, Acyrthosiphon pisum. J Insect Physiol 1997;43:615ŌĆō620.


52. Richter K, Peschke E, Peschke D. A neuroendocrine releasing effect of melatonin in the brain of an insect, Periplaneta americana (L.). J Pineal Res 2000;28:129ŌĆō135.


53. Yamano H, Watari W, Arai T, Takeda M. Melatonin in drinking water influences a circadian rhythm of locomotor activity in the house cricket, Acheta domesticus. J Insect Physiol 2001;47:943ŌĆō949.

54. Richter K, Peschke E, Peschke D. Effect of melatonin on the release of prothoracicotropic hormone from the brain of Periplaneta americana (Blattodea: Blattidae). Eur J Entomol 1999;96:341ŌĆō345.
55. Subala SPRR, Shivakumar MS. Changes in light and dark periods affect the arylalkylamine N-acetyl transferase, melatonin activities and redox status in the head and hemolymph of nocturnal insect Spodoptera litura. Biol Rhythm Res 2017;49:13ŌĆō28.

56. Freelancea BC, Leone L, Anderson CR, Jones TM. A method for paraffin sectioning and identification of indoleamines in the brain of insects with a sclerotized cuticle. J Histotechnol 2017;40:66ŌĆō72.