Association Between Benzodiazepines and Dementia in South Korea: A Nation-Wide Study
Article information
Abstract
Objective
Benzodiazepines are a widely used class of medications for anxiety, depression, and insomnia. Despite their common use, concerns remain about memory problems with benzodiazepines. Despite the growing financial and social burden of dementia, inconsistent results persist regarding the association between benzodiazepines and dementia. Therefore, we aimed to evaluate the association between benzodiazepines and dementia in Korea.
Methods
Diagnostic and prescription information from the Health Insurance Review and Assessment (HIRA) database in South Korea between 2009 and 2014 was utilized. The dementia group included people who were diagnosed with a dementia code and received one or more prescriptions for dementia. A total of 68,241 participants with dementia and 341,205 control participants were matched. Possible confounders, such as major medical and psychiatric disorders, were adjusted, and multivariate logistic regression was conducted to assess the association between benzodiazepines and dementia.
Results
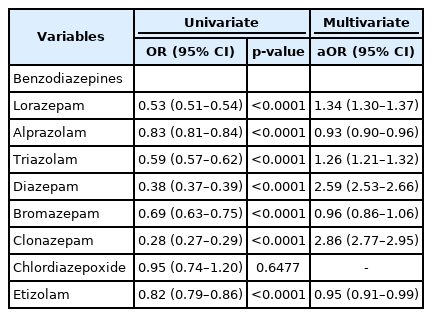
The highest odds ratio (OR) for dementia was noted for clonazepam (OR=2.86, 95% confidence interval [CI]=2.77–2.95) followed by those for diazepam (OR=2.60, 95% CI=2.53–2.66), lorazepam (OR=1.34, 95% CI=1.30–1.37), and triazolam (OR=1.26, 95% CI=1.21–1.32).
Conclusion
Overall, relatively long-acting benzodiazepines, such as clonazepam, diazepam, and lorazepam, were associated with the incidence of dementia. Triazolam, which is approved for insomnia, was also significantly associated with dementia. Individuals who are prescribed with benzodiazepines should be cautious regarding memory loss and dementia. Further studies are needed to confirm the temporal and biological causality between benzodiazepines and dementia.
INTRODUCTION
Benzodiazepines are a widely used class of drugs that are used for treating anxiety, depression, and insomnia [1-3]. Benzodiazepines are excellent alleviators of stress and anxiety, by enhancing the effects of gamma-aminobutyric acid [4]. Additionally, benzodiazepines are frequently used to reduce rapid eye movement (REM) sleep and sleep onset latency [5,6], which aids in restoration of psychological function. In South Korea, triazolam, flunitrazepam, and brotizolam are the approved benzodiazepines by the Korean Food and Drug Administration (KFDA) for managing short-term insomnia [7]. Due to their high potential for dependency, they are advised for up to 4 weeks in the treatment of insomnia. Additionally, other benzodiazepines, such as clonazepam, diazepam, lorazepam, and etizolam are not approved for insomnia by the KFDA but are frequently used off-label for treating insomnia in clinical settings. Therefore, it is challenging for both patients and healthcare providers to prescribe benzodiazepines optimally.
Aside from their diverse indications, benzodiazepines are known to result in problems with long-term use [8]. With higher dosages and/or long-term use, benzodiazepines are known to have an abuse potential and result in respiratory depression, falls, and withdrawal [9-12]. Additionally, problems with attention and memory, such as impaired daytime performance, anterograde amnesia, and memory disturbances have also been reported [13-16]. Due to their potential for memory impairment, many have questioned whether memory problems due to benzodiazepines lead to dementia.
Dementia is a neurocognitive disorder that is characterized by loss of cognition in multiple cognitive domains along with deficits in social and/or occupational functions [17]. There are numerous subtypes of dementia, such as Alzheimer’s disease (AD), vascular dementia, dementia of Lewy bodies, and Parkinson’s disease dementia. Although there have been pharmacological advancements regarding the optimal cure for AD over the years, AD remains a major public health issue worldwide.
Due to public concerns regarding the social and financial burden of dementia, numerous experts have investigated the risk of dementia with benzodiazepines. Previous studies have evaluated the short-term memory effects of benzodiazepines; however, the detrimental effects of long-term use of benzodiazepines remain inconsistent. Some previous studies have suggested that benzodiazepines are associated with the incidence of neurocognitive disorders [18-21], while other studies have reported mixed or even potentially protective effects [22-26]. Therefore, in the current study, we evaluated the association between benzodiazepines and dementia by comparing the effects of each benzodiazepine in a Korean nationwide sample.
METHODS
Data collection
We used the Health Insurance Review and Assessment (HIRA) database to collect data regarding the diagnosis codes and medications that were prescribed. HIRA, known as the National Health Insurance (NHI) data, includes the demographic, clinical, and prescription data of nearly 98% of the South Korean population. HIRA data are generated during the reimbursement of healthcare providers under NHI and contain clinical information [27]. This information ranges from general demographic data to data regarding the diagnosis, procedures, and outpatient prescriptions, such as drug codes, coverage, and quantity of prescribed medications.
We collected the diagnostic and prescription data between January 1, 2009, and December 31, 2014, from the HIRA database. The clinical data were collected from the medical institutions that were eligible for insurance benefits. A total of 296,787,595 participants were registered in the HIRA with national health insurance during 2009–2014. In this study, an operational definition of the dementia group of patients was used for a reliable analysis. We defined the dementia group as the group of patients who were diagnosed with dementia-related diagnostic codes and were prescribed anti-dementia drugs more than once.
Subjects
The following diagnostic codes for dementia from the Korean Informative Classification of Disease (KOICD) were used: F00, F01, F02, F03, G30, and G31. Anti-dementia medications included the following drugs that were prescribed more than once during the observation period: donepezil (1486XXXXX), rivastigmine (2245XXXXX), galantamin (3852XXXXX), and memantine (1900XXXXX). The control group was defined as patients who were not diagnosed with any of the dementia codes and did not receive the aforementioned dementia medications. The dementia and control groups were matched by age and sex at a ratio of 1:5. Additionally, insurance classification codes were adjusted. Current study was reviewed and approved by the Institutional Review Board of the Korea University Anam Hospital (IRB no. ED 15238), with a waiver of documentation of informed consent.
Clinical information
The following three main categories of clinical information were included in our study: benzodiazepines prescribed, medical disorders, and psychiatric disorders. Benzodiazepines included lorazepam, alprazolam, triazolam, diazepam, bromazepam, clonazepam, chlordiazepoxide, and etizolam. Major medical disorders included malignancy, heart diseases, cerebrovascular disease, pneumonia, diabetes, chronic lower respiratory disorders, liver diseases, and hypertension. Psychiatric disorders included schizophrenia spectrum disorders, major depressive disorder, bipolar disorder, anxiety disorder, substance use disorder, insomnia disorder, and other mental disorders.
Statistical analysis
The primary outcome was the risk of dementia associated with various types of benzodiazepines. All patients who were prescribed benzodiazepines more than once during the observational period were included in the analysis. To identify potential confounding variables, the association between major medical and psychiatric disorders and dementia was explored using a univariate logistic regression. A variable with p-value <0.1 was considered a potential confounding variable and corrected in the main analysis. Univariate and multivariate logistic regression analyses were performed to determine the association between each type of benzodiazepine and dementia. We calculated the 95% confidence interval (CI) using statistical significance of p-value <0.05. Data were analyzed using SPSS (ver. 26.0; IBM Corp., Armonk, NY, USA).
RESULTS
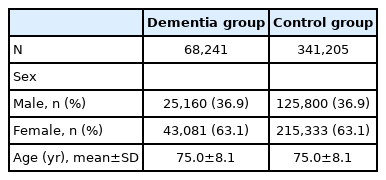
A total of 68,241 patients were identified with a diagnosis of dementia and were prescribed anti-dementia drugs more than once. Additionally, 341,205 control patients were matched for age and sex. The mean age and standard deviation of the participants was 75.0 (±8.1) years. The characteristics of the patient and control groups are outlined in Table 1.
Using univariate logistic regression, we investigated potential confounders among major medical and psychiatric disorders for an association between benzodiazepines and dementia. Univariate analysis revealed a statistically significant association between all major medical disorders and dementia. In addition, all psychiatric disorders also had significant association with incidence of dementia (Table 2).
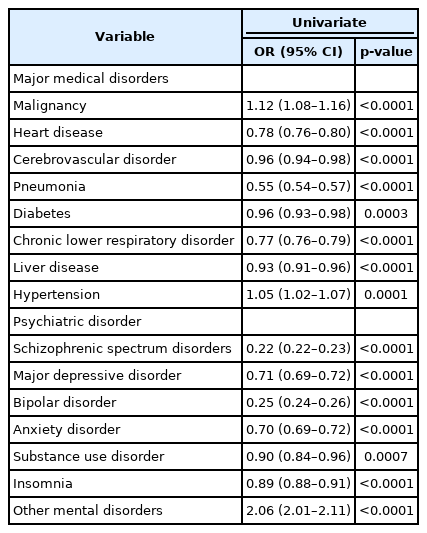
Univariate logistic regression analysis of dementia with major medical and psychiatric disorders for evaluating potential confounding factors
For a more reliable evaluation of the association between dementia and each benzodiazepine, we adjusted for potential confounding medical and psychiatric factors based on the results of each regression analysis mentioned above. A p-value <0.1 was considered a potential confounding factor and adjustments were made in the main analysis. Finally, using multivariate logistic regression, the OR for each benzodiazepine was calculated (Table 3). Clonazepam had the highest OR for dementia (OR=2.86, 95% CI=2.77–2.95) followed by diazepam (OR=2.60, 95% CI=2.53–2.66), lorazepam (OR=1.34, 95% CI=1.30–1.37), and triazolam (OR=1.26, 95% CI=1.21–1.32). The OR for bromazepam was 0.96 (95% CI=0.86–1.06), while those for short-acting benzodiazepines, such as etizolam and alprazolam, were 0.95 (95% CI= 0.91–0.99) and 0.93 (95% CI=0.90–0.96), respectively. Chlordiazepoxide was not found to have a statistically significant association with the incidence of dementia.
DISCUSSION
In this study, we evaluated the association between benzodiazepines and the risk of dementia in the South Korean population. The database used in this study was based on nationwide healthcare services in South Korea. We defined patients who were 1) diagnosed with a dementia diagnostic code and 2) prescribed anti-dementia medications more than once as the dementia group. Our results suggest that relatively long-acting benzodiazepines, such as clonazepam and diazepam are associated with the incidence of dementia. The findings of this study are consistent with those of previous studies that examined the relationship between benzodiazepines and dementia [18,19,28]. A case-control study that used health insurance data in Taiwan found an increased risk of dementia in patients who received long-term benzodiazepines (>6 months) [8].
Although the current study did not directly investigate the temporal relationship or causality between benzodiazepines and dementia risk using a cohort study design, our findings support the hypothesis that benzodiazepines, especially the long-acting and intermediate-acting benzodiazepines, are associated with the incidence of dementia. Additionally, our study specifically highlighted the benzodiazepines with strong associations with dementia using OR. Consequently, we identified that relatively longer-acting benzodiazepines are strongly associated with dementia. Since the etiology of dementia is heterogeneous, the biological mechanisms related to dementia and benzodiazepines remain unclear, which is a scope for future studies to investigate.
Certain points should be considered when interpreting the results of this study. It is important to determine the purpose of benzodiazepines in Korea. Since some benzodiazepines, such as triazolam, are approved for treating short-term insomnia [7], we have to consider whether the patients who were prescribed these benzodiazepines have only insomnia or have insomnia as part of a neurodegenerative process. Furthermore, compared to other benzodiazepines with significant associations in this study, the results of triazolam—which is used for insomnia but known to have serious dependence problems—are clinically noteworthy.
Unlike triazolam, other significantly different, relatively long-acting benzodiazepines are prescribed for various purposes in clinical practice. For example, clonazepam is frequently used in REM sleep behavior disorder, which is often found in Lewy body dementia [29]. Diazepam is also used for anxiety, which can be confused as psychomotor agitation in behavioral and psychological symptoms of dementia in clinical settings [30,31]. Therefore, when interpreting our results, caution is needed to determine whether these benzodiazepines are prescribed before dementia or as part of treatment for symptoms of dementia. Furthermore, since we could not screen whether individuals who were prescribed benzodiazepines were already prone to neurodegeneration, further investigations are warranted.
Our study has some limitations. First, the current study was a case-control study, which limits drawing any conclusions about the temporal or causal relationship between benzodiazepines and dementia. This was a limitation of the data available; therefore, this aspect should be improved in the future. Second, while collecting the dementia diagnoses, there is a potential risk for misclassification. To solve this issue, in this study, the group that received not only a diagnosis of dementia but also a prescription for dementia medications was defined as the dementia group. Third, lifestyle and family history factors that may be closely related to the onset of dementia were not included in the analysis. Confounding factors, such as alcohol consumption or family history of dementia can also result in dementia.
In conclusion, this retrospective case-control study suggests that benzodiazepines are associated with the incidence of dementia. Individuals who are prescribed benzodiazepines should be cautious about the risk of memory impairment and dementia. Patients who are prescribed benzodiazepines, especially elderly patients, should be closely evaluated and followed up for any memory concerns and the onset of dementia in clinical practice. Future studies are needed to confirm our findings by investigating the causality of benzodiazepines and the development of dementia as well as elucidate the possible biological mechanisms that underly the effects of long-term benzodiazepines on memory and dementia.
Notes
Funding Statement
This work was supported by National Research Foundation (NRF) of Korea grants funded by the Ministry of Science and Information and Communications Technology (MSIT), Government of Korea (NRF-2020R1C1C1007463 and NRF- 2021R1A5A8032895) (Dr. Cho).
The authors have no potential conflicts of interest to disclose.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Heon-Jeong Lee. Data curation: Chul-Hyun Cho, Ji Won Yeom. Formal analysis: Chul-Hyun Cho, Heon-Jeong Lee. Investigation: Tai hui Sun. Methodology: Chul-Hyun Cho, Tai hui Sun. Supervision: Heon-Jeong Lee. Writing—original draft: Tai hui Sun. Writing—review & editing: Chul-Hyun Cho, Heon-Jeong Lee.