INTRODUCTION
Circadian rhythms exist in almost all organisms in nature and enable adaptation to appropriate temporal niches by regulating physiological variables such as heart rate, hormone secretions, neurotransmitter secretions, biochemical pathways, cognition, and many other processes through interaction with rhythmically transcribing clock genes that are regulated by photic and nonphotic factors known as zeitgebers [
1,
2]. Frequent trans-meridian travel, rotating shift work schedules, and inconsistent sleep habits result in a loss of synchronization between the endogenous circadian timing system and the external environment in modern lives [
3]. The synchronization of endogenous circadian timing and external signals is critical for sustaining normal physiology.
This physiological readjustment takes a number of days, during which activity onsets and offsets alter progressively in a sequence of stages known as transient cycles. Transient cycles may be studied by recording the locomotor activity rhythm and plotting it as an actogram. The acquisition, organization, usage, and modification of knowledge about spatial surroundings is the focus of spatial memory. To navigate around a familiar environment, a person’s spatial memory is necessary, much as a rodent’s spatial memory is required to remember the location of food or a platform at the end of a maze [
4]. The circadian system is connected to and governs memory acquisition, processing, and access in the temporal lobe, specifically the hippocampus [
5]. This process has been found to be significantly influenced by various light-dark patterns, jetlag, light exposure during rest periods, and other aspects. Daily exposure to various light-dark cycles in the population has a detrimental impact on memory and results in a number of health issues. Chronic shift exposure causes decreased oxygen availability at the tissue level, which results in the formation of superoxide radicals, which in turn create hydroxyl and peroxynitrite radicals in a chain reaction [
3]. Under prolonged exposure, the antioxidants and free radical scavenging enzyme system play a critical role in quenching the free radicals produced as a consequence of numerous metabolic activities. Frequent transmeridian travel or shift work may need more potent antioxidant defence systems for quenching the free radicals produced by light-dark shifts, altering the activity of antioxidant enzymes such as superoxide dismutase (SOD) and catalase (CAT) [
6]. The cumulative effect of an impaired antioxidant system and increased free radical generation leads to a change in locomotor activity, memory recall, antioxidant enzymes, lipid peroxidation (LPO), and reactive oxygen species (ROS) level [
6] and altered clock gene
PER1 and memory regulating
BDNF genes expression.
The root extract of
Withania somnifera is used as a popular herbal drug in Ayurvedic medicine and has been used traditionally as a tonic and nootropic agent. It improves cognitive function and increases mental retention ability after diabetes or scopolamine-induced memory loss [
7,
8]. Methanolic extract of the
Withania root has a strong relationship with dendritic extension and dendritic arborization [
9]. In normal cortical neurons, withanolide-A (WA), the primary active component isolated from the root of
Withania somnifera, induces axonal outgrowth [
10]. In the present study, we investigated the effect of WA on the re-entrainment of circadian locomotor activity rhythm, memory dysfunction, gene expression and antioxidant enzyme system of chronic jetlag exposed mice.
METHODS
Subjects
Adult male mice, Mus musculus (AKR strain) of about 3 months old were used for this experiment. The mice were maintained in the animal house under the 12:12 h light-dark cycle with a temperature of 25°C±2°C. The food pellets and water were provided ad libitum. An electronic timer (Larsen and Toubro, Mumbai, India) was used to maintain the light-dark cycle. The light intensity at the cage level was about 150 lx during the light phase and 0 lx during the dark phase. A red-filtered torch light (<1 lx) was used during dark hours for maintenance, for which visits were made at random times. The Institutional Animal Ethical Committee norms of the University (Dean/13–14/CAEC/185), the recommended ethical considerations for biological rhythm research [
11], and the specified maintenance and analysis protocols by Jud et al. [
12] were followed for this investigation.
Experimental groups
Animals were divided into different sets, each set having four groups: 1) normal group (LD) exposed to normal 12:12 h light-dark condition throughout the experimental protocol; 2) control group (chronic jetlag, CJL) exposed to chronic 9 h advance-delay light-dark schedule; 3) a vehicle-treated group (VEH) (0.5% gum arabic, Himedia, Mumbai, Maharashtra, India), which was exposed to chronic 9 h advance-delay light-dark schedule and treated with a single oral dose of the vehicle per day; and 4) WA-treated group (10 µmol/kg/day, Cayman, Ann Arbor, MI, USA) given a single oral dose each day and exposed to chronic light-dark shift after the pre-dosing period. All the doses were administered orally for 13 consecutive days at around ZT15 (zeitgeber time 15) and the simulated shift work/jetlag was evoked on the day following the last dose.
In Set 1, the mice were assigned randomly to four groups (n=6/group), and circadian locomotor activity rhythm was recorded and data was analyzed using ClockLab analysis software (Coulbourn Instruments, Whitehall, PA, USA).
In Set 2, the mice were assigned randomly to four groups (n=8/group), and short-term spatial memory was studied using the Y-maze.
In Set 3, the mice were assigned randomly to four groups (n=8/group), and long-term spatial memory was studied using a Barnes maze.
In Set 4, the mice were assigned randomly to four groups (n=6/group), and PER1 and BDNF genes expression was studied in hippocampus and suprachiasmatic nuclei (SNC) tissues by reverse transcription-quantitative polymerase chain reaction (RT-qPCR).
In Set 5, the mice were assigned randomly to four groups (n=6/group), and the antioxidant enzymes SOD and CAT, as well as ROS and LPO levels were studied in the hippocampus.
Measures
Locomotor activity recording and simulation of chronic jetlag/shift work
Set-1 included 24 adult mice divided into four groups. LD group was undisturbed. CJL group was exposed to chronic light-dark shift parallelly with other VEH and WA-treated groups. All the doses were administered orally for 13 consecutive days at around ZT15, and the chronic jetlag was simulated on the day following the last dose by 9 h advancing and 9 h delaying light-dark cycle continuously three times from the original light-dark cycle.
Initially, mice were housed in a chronocubicle under LD 12:12 h cycle (06:00 h to 18:00 h light, 18:00 h to 06:00 h dark) for 10 days of baseline circadian activity recording. An electronic timer (Larsen and Toubro, Mumbai, India) was used to maintain the light-dark cycle. The ClockLab (Coulbourn) setup, with an ACT553 breakout box (7-channel) and a CL-200 interface, was used to record and analyze voluntary wheel-running activity. Individually-housed mice were allowed unlimited access to individual running wheels (9.525 cm diameter) inside their cages (36 cm×20 cm×14 cm). Subsequently, the light-dark cycle was shifted alternatively, 9 h advance for 2 days and 9 h delay for 2 days. The same sequence was repeated three times continuously then mice were allowed to re-entrain to the normal 12:12 h LD schedule. Subsequently, the locomotor activity records (Actograms) were analyzed with ClockLab software (version 2.63, Actimetrics,
https://actimetrics.com/products/clocklab/).
Study of short-term memory using Y-maze
Set-2 consisted of 32 mice divided into four groups for the Ymaze behavioral test. Y-maze has been extensively used to quantify working memory and anxiety in rodents. The test explores the innate natural exploratory tendency or behavior of rodents. The maze apparatus contains three identical arms (30 cm in length). One arm is allowed to remain novel to the rodent under test (remains closed during 1st trial), and out of the remaining two arms, one is chosen as the starting arm where the animal is positioned at the beginning of the trial. Each mouse of all groups (LD, CJL, VEH, and WA-treated groups) was given two trials; in the first trial, the novel arm was blocked and the mouse was allowed to explore the other two arms for 3 min. In the second trial, mice were freely allowed to explore all three arms of Y-maze for 3 min. The inter-trial interval was around 30 min between two trials for the same mouse.
Study of long-term memory using Barnes maze
Set-3 consisted of 32 mice divided into four groups for Barnes maze behavioral test. A circular, black platform, 90 cm in diameter, with a 90 cm high pedestal, with 20 equidistant holes and an escape box that could be placed below any of the holes, served as the Barnes maze. Visual cues were provided in the experimental chamber in such a way that three discrete visual cues in the form of different geometric shapes (square, circle, and triangle) of paper were placed approximately 1 m away from the edge of the maze. The mice were exposed to negative reinforcement during the task in the form of bright light (450 lx) which motivated the mice to move to the escape box. All sessions were recorded using a video camera placed above the apparatus and analyzed with video tracking software (SMART, version 3.0.06; Panlab, Harvard Apparatus, Holliston, MA, USA).
Briefly, the test was divided into three phases: habituation, training, and probe trial. The first day was the habituation period mice were allowed to freely explore the maze apparatus for 3 min. The training was performed for 3 consecutive days followed by habituation day; where each mouse was given 3 trials of 180 sec per day with an inter-trial difference of a minimum of 15 min, and the time taken to find the escape box was noted. Habituation and learning were done during the last 4 days of dose administration. Each mouse of all the groups (LD, CJL, VEH, and WA) was similarly given training. Except for the LD group, all other three groups were exposed to the simulated chronic jetlag. A probe test (90 sec) was done after chronic jetlag respectively to test the retention power of mice that were trained during the training phase before the chronic jetlag simulation. All the training and testing protocols were carried out at the same zeitgeber time. After each trial, the maze was wiped down with 70% ethanol and allowed to dry to remove any olfactory cues.
RT-qPCR
Briefly, total RNA was isolated from mice’s hippocampus and suprachiasmatic nuclei from each group (n=6/group) using a Trizol (Invitrogen, Carlsbad, CA, USA). For cDNA synthesis, 5 μg of RNA from each group was reverse transcribed. The cDNA was used as a template for RT-qPCR amplification using specific following primers for PER1 and BDNF genes. The endogenous control GAPDH was used to normalize the quantification of the mRNA target.
Biochemical analysis
After the pre-dose administration of WA/VEH and the chronic jetlag exposure, the mice were euthanized by cervical dislocation. The hippocampus region was then isolated from the mouse brain, pooled together separately in LD, CJL, VEH, and WA, and homogenized in 0.1 M phosphate buffer. The homogenate was subsequently centrifuged at 12,000 rpm for 15 min at 4°C. The supernatant fraction was collected and stored at -80°C until analysis. The protein concentration of samples was quantified using the Bradford method at 595 nm and bovine serum albumin as standard (Bradford, 1976). All reactions were performed spectrophotometrically (Biospectrometer, Eppendorf, Hamburg, Germany) in triplicates.
Total ROS
The ROS level generated in mice was detected using the non-polar fluorescent probe dichloro-dihydro-fluorescein diacetate (DCFH-DA). A highly fluorescent compound, dichlorofluorescein (DCF), is formed while interacting with ROS after converting DCFH-DA to a polar derivative. Briefly, the 20 µL of homogenate, 100 µL of PBS, and 8.3 µL of DCFH-DA from stock solution (10 mg/mL; dissolved in DMSO) were added to each 96-well plate. Then incubation was done at 37°C for 30 min. After that, the fluorescence intensity was determined at 485 nm for excitation and 530 nm for emission wavelength using a multimode reader (Synergy HI, Biotek Instrument; Winooski, VT, USA).
Antioxidant stress response
Lipid peroxidation was detected as described by Ohkawa et al. [
13] where malondialdehyde (MDA) reacts with thiobarbituric acid (TBA) and gives a colored complex which is detected by using spectrofluorimetric analysis. The MDA levels were measured as nmol/mg protein. SOD activity was estimated following the method by Das et al. [
14]. The absorbance was taken at 543 nm using a spectrophotometer. The enzymatic activity was expressed in units/mg of protein. The CAT activity was measured according to Aebi [
15]. The change in the absorbance due to H2O2 was measured in the hippocampal tissues. The CAT activity was expressed in units/mg of protein.
Statistical analyses
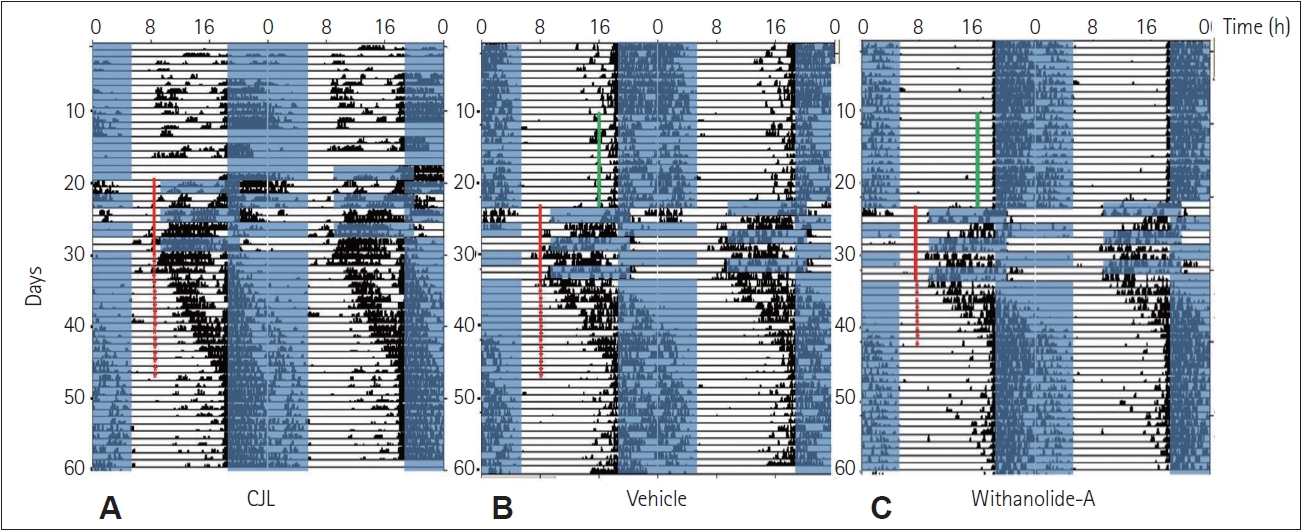
Circadian rhythms in wheel-running behavior were depicted as double plotted actograms in 6 min bins, using ClockLab software (Coulbourn, version 2.63). The mean number of transient cycles (days taken to re-entrain to the advanced or delayed light-dark schedules) was compared for the significant difference between CJL, VEH, and WA-treated groups using one-way analysis of variance (ANOVA). Transient cycles were measured by visual observation (marked by the red stars on representative actograms of each group) for each mouse in each group. Videos recorded for the experiments involving the Barnes maze and Y-maze were analyzed using Panlab Smart version 3.0.06 (Harvard Apparatus), and the various parameters obtained from the experimental groups were examined for significant differences using the one-way ANOVA followed by a Tukey’s honestly significant difference (HSD) post hoc test. Biochemical assays and gene expression data were also tested for significance by one-way ANOVA followed by a Tukey’s HSD post hoc test. The data were graphically represented as the mean± standard error. The results were considered statistically significant with p<0.05.
DISCUSSION
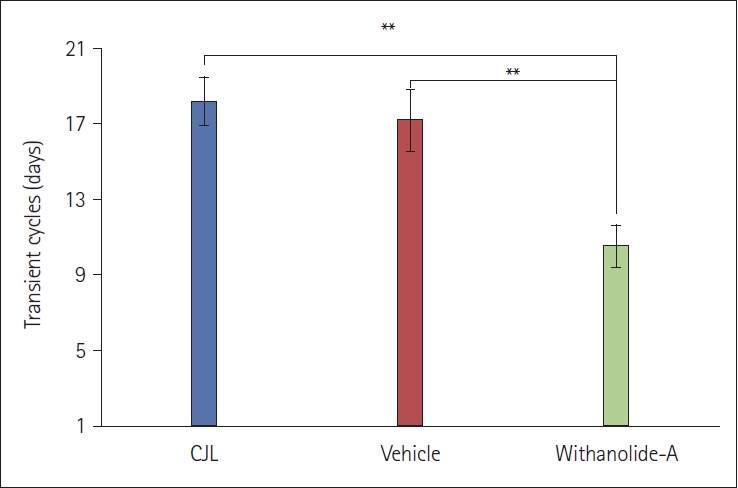
The effects of WA on the re-entrainment of circadian locomotor activity rhythm of mice subjected to 9 h chronic simulated jetlag/shift work were studied. The actograms and the number of transients indicated that WA-treated mice re-entrain to chronic 9 h light-dark shift faster compared to CJL and VEH groups (
Figures 1 and
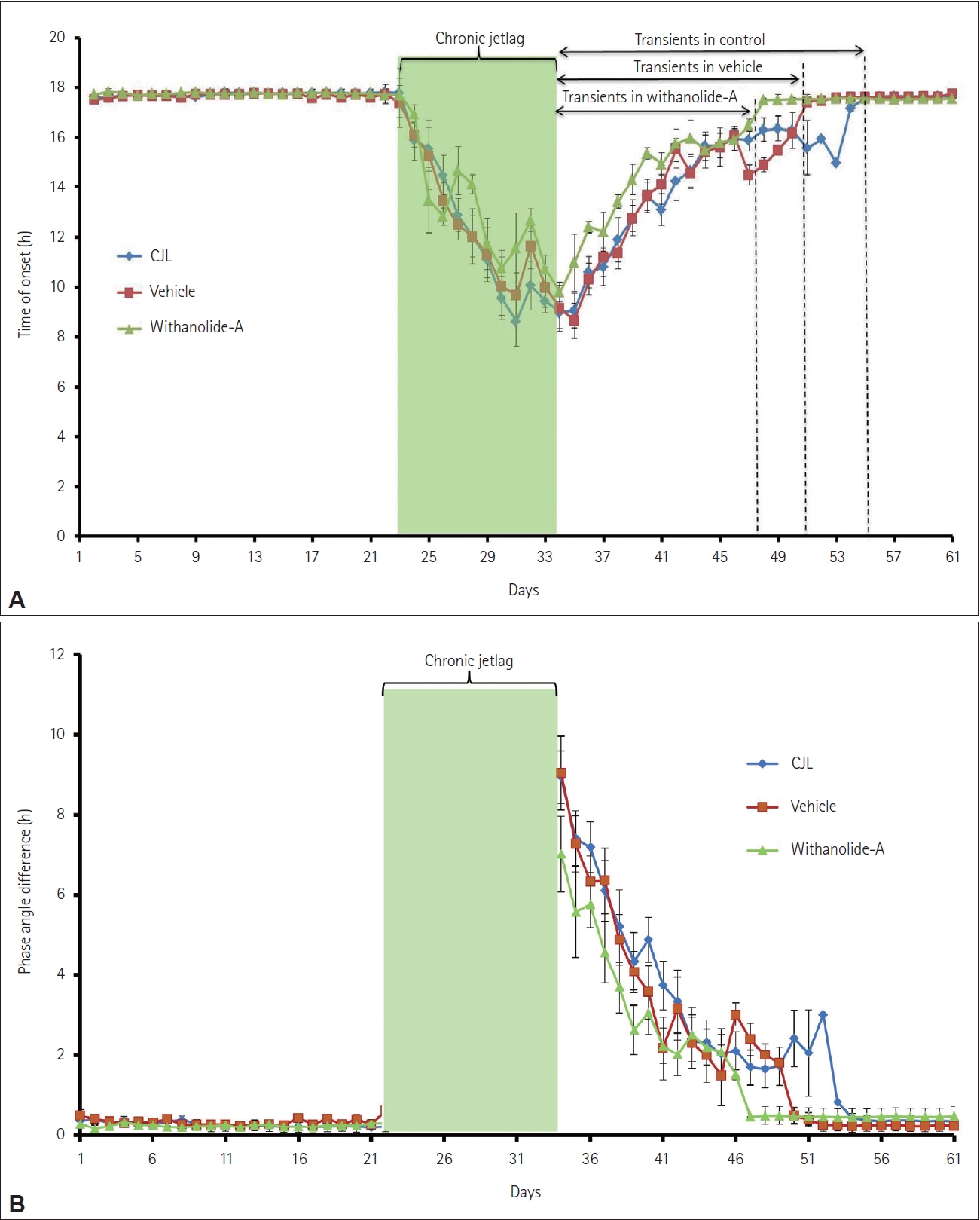
2). The control (CJL) group mice remain non-synchronized for a longer period after chronic jetlag exposure and took comparatively more transient cycles among the exposed groups to synchronize the circadian rhythm. The CJL and VEH group mice took almost the same number of transient cycles showing no effect of gum acacia used as a vector solvent for the chemical. This showed that chronic 9 h advance-delay shift is potent to create physiological stress and/or cognitive damage. This is further clearly shown by the onset of activity plot (
Figure 3A) extrapolating clearly how activity onset changes during and post CJL in all the groups. This showed that activity onset gets synchronized faster in the WA-treated group compared to the other two CJL and VEH groups after jetlag exposure. Chronic light-dark shift further showed changes created in phase angle difference in all the three exposed groups which can be seen in
Figure 3B. It showed that WA-treated group mice synchronize their phase angle faster compared to CJL and VEH groups.
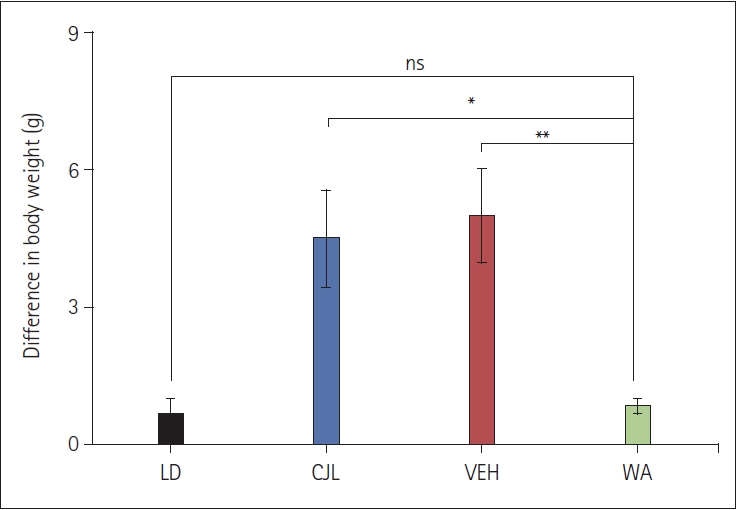
The simulated jetlag has a potent effect on the body weight of mice that were exposed to chronic 9 h light-dark shift (
Figure 4), leading to a gain in the body weight of mice which may be because of an increase in rest condition or lethargic activity of mice occurred due to disturbance in body physiology and behavior, an effect of frequent changes in light-dark shifts. Additionally, it may increase stress, which ultimately leads to mice feeding more, resulting in an increase in body weight.
Chronic jetlag exposure leads to disturbance causing decreased hippocampal and SCN neurogenesis and subsequently poor cognitive performance in terms of long-term potentiation of memories [
16,
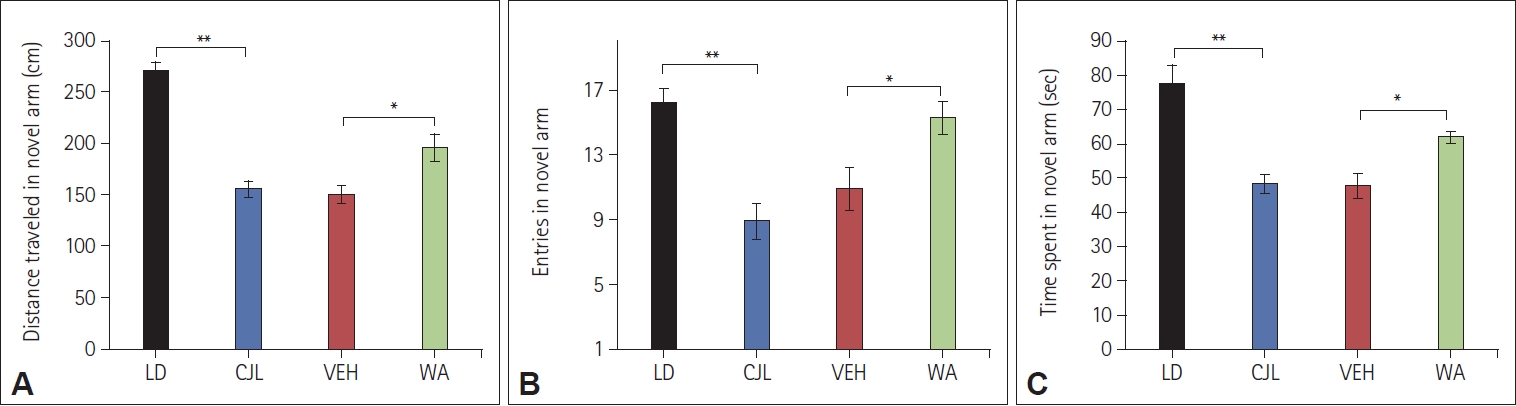
17]. Thus, further short-term and long-term spatial learning and memory were studied. The effect of simulated chronic jetlag on short-term memory was studied by using the Y-maze exploratory behavior of mice that were given a pre-trial before the test trial. The results showed a significant decrease in distance traveled in the novel arm, the number of entries in the novel arm, and time spent in the novel arm significantly decreases in all three groups (CJL, VEH, and WA treated) compared to the LD group mice during the second trial of Y-maze behavioral test (
Figure 5). This result further showed that chronic jetlag leads to a disturbance in short-term memory retention in all the mice exposed to it but WA-treated mice showed better performance compared to CJL and VEH groups. Jetlag simulation reduced hippocampal neural precursor cell proliferation and impaired spatial memory performance in adult C57Bl6 mice during water maze test. A study on mice exposed to photoperiod alterations over a 3-week period and whose dark periods were shortened every third day by 6 hours found that melatonin prevented both cellular and cognitive deficits. Our work showed similarity with this work suggesting that WA may also be acting as a neuroprotective agent, supported by the Y maze and Barnes maze behavioral results as shown in
Figures 5 and
7 [
18] and discussed further.
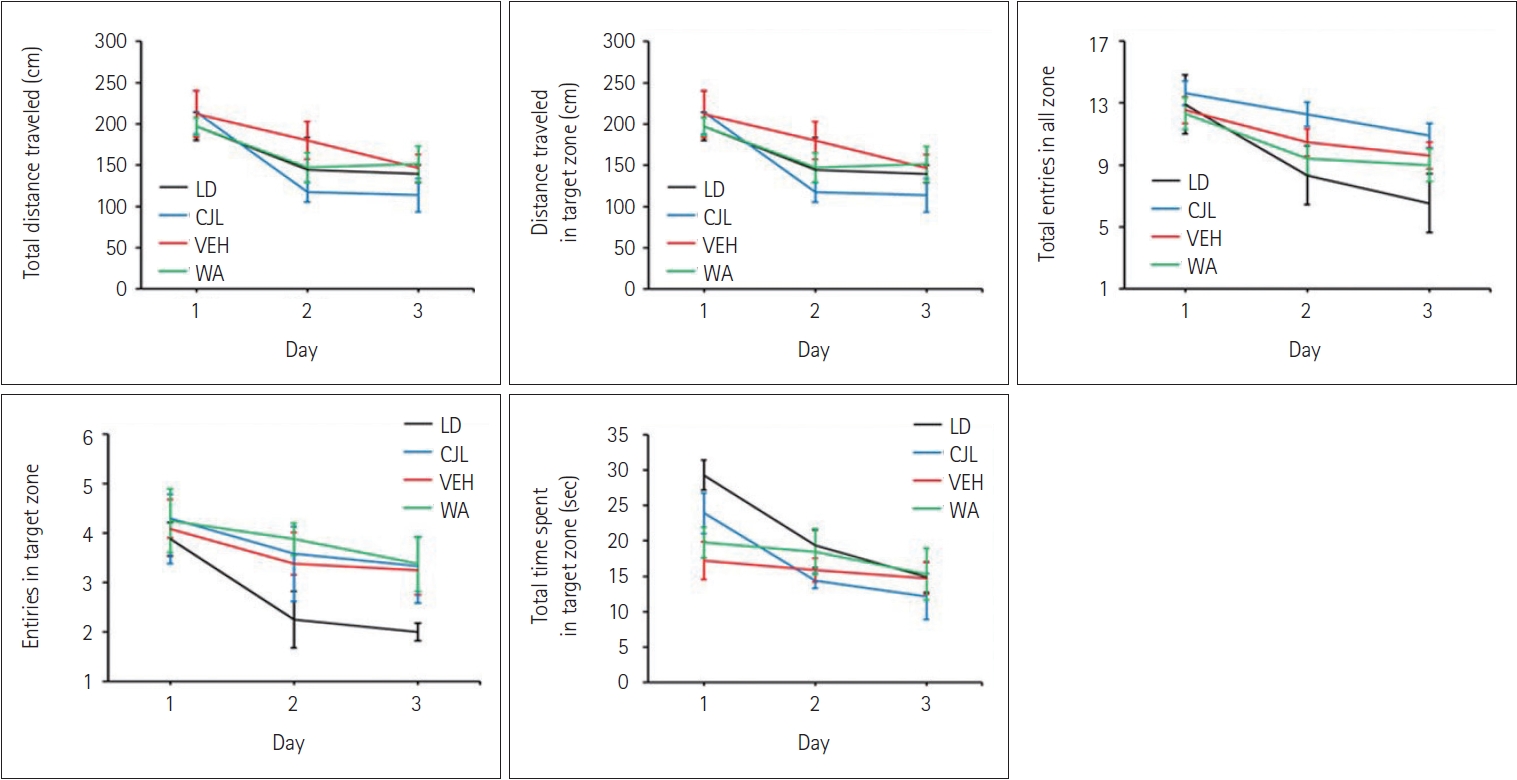
The neuroprotective property of WA was studied in a long-term memory test which was performed with the help of the Barnes maze behavioral apparatus. Mice demonstrated effective learning patterns during the training period, which can be seen by the line graph of total distance traveled, distance traveled in a target zone, total entries in all zones, entries in the target zone, and total time spent in a target zone (
Figure 6). During the memory test, WA-treated mice performed better compared to CJL and VEH treated groups. Total distance traveled and distance traveled in the target zone both increased in CJL, VEH, and WA-treated mice compared to the normal LD group (
Figure 7). Although WA-treated mice do not show significant differences compared to the LD group, unlike CJL and VEH groups which differ significantly from LD group. Similarly, entries in all zones and entries in the target zone showed significant differences among the groups. Although chronic jetlag leads to an increase in total entries and entries in the target zone compared to normal LD group mice. Further time spent in the target zone was also increased after chronic jetlag which showed the deleterious effect of chronic jetlag on recall or memory. Together, these results showed that chronic jetlag has a deleterious effect on cognition [
19,
20] and WA-treated mice performed better in both short-term and long-term memory tests and may be acting as a neuroprotective agent through some molecular link. These findings suggested that WA may have the property to be used as a neuroprotective agent and can be used for other clinical studies.
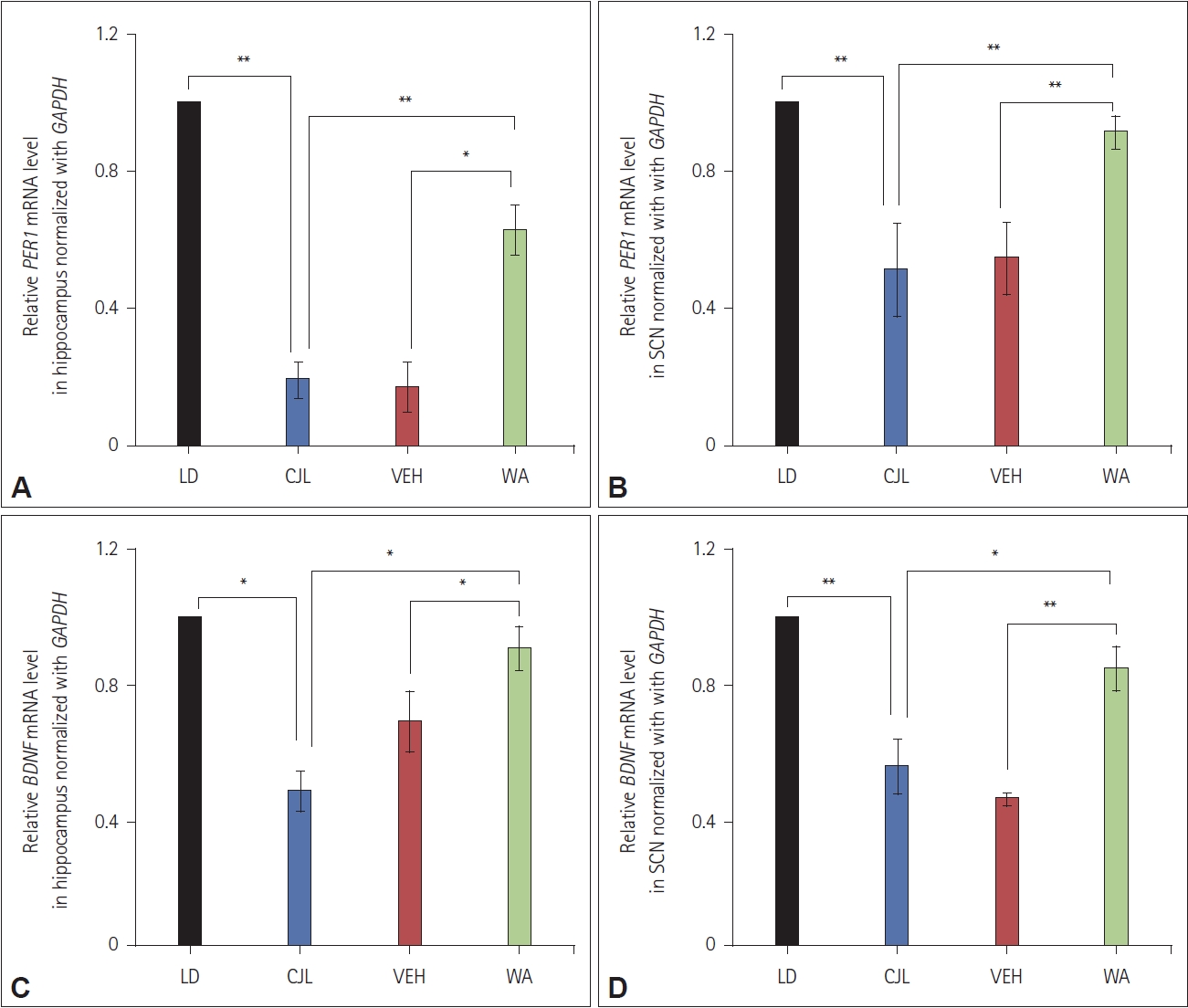
In addition, the concomitant decreases of
BDNF levels were seen in chronic jetlag exposed mice both in the hippocampus (
Figure 8A and C) and SCN regions (
Figure 8B and D) of the brain which was studied through RT-qPCR (expression normalized to
GAPDH). These results further supported the behavioral results, which showed chronic jetlag has a deleterious effect on learning and memory which may be because of a downregulation of
BDNF gene expression. The results also demonstrated that WA treatment tries to positively modulate the expression of the
BDNF gene as seen by elevated expression levels in the hippocampus and SCN. Mice subjected to chronic jetlag showed a substantial reduction in
BDNF gene expression at the hippocampus and SCN compared to the normal LD group.
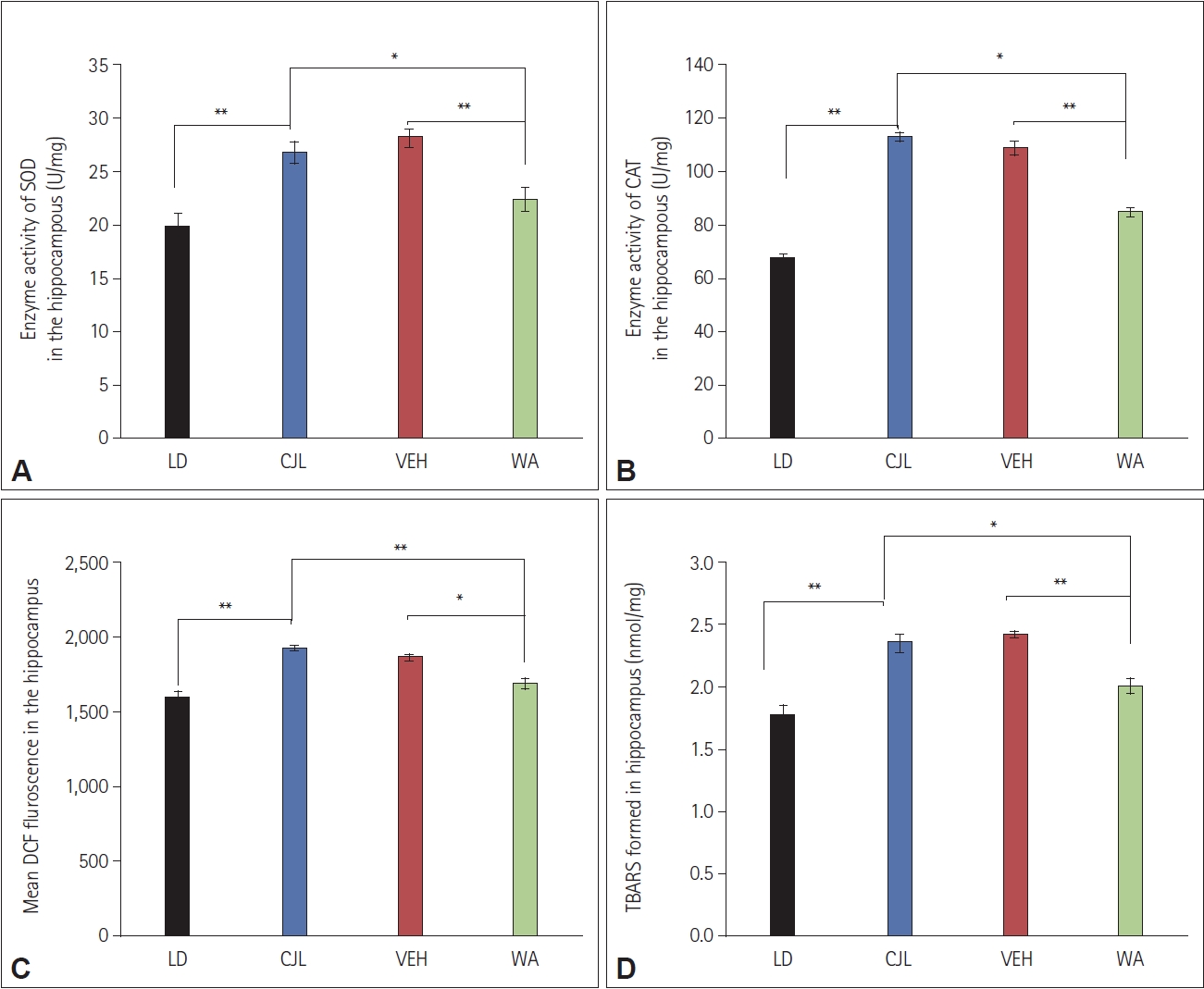
Biochemical estimation of the antioxidant enzyme showed that chronic jetlag leads to an increase in activity of SOD and CAT (
Figure 9A and B) implicating that jetlag causes an increase in the production of harmful oxygen radicles and this enzyme system actively participates to balance these harmful radicles, regulating stress level. WA-treated group showed a significant difference in SOD and CAT enzymes activity compared to CJL and VEH groups. The WA-treated group showed the potent effect of treatment on harmful oxygen radicle production, showing its protective role [
6]. The ROS level was also elevated due to chronic jetlag exposure. A significant difference was seen in the WAtreated group and CJL, WA-treated group and VEH (
Figure 9C) and lipid peroxidation was also found to increase in the chronic jetlag exposed group compared to LD. A significant difference was seen in the WA-treated group and CJL and WA with VEH (
Figure 9D) [
6].
The present study showed the dual effects of WA on the acceleration of re-entrainment of the circadian clock and its neuroprotective effect on the post-circadian rhythm of chronic shift has not been carried out before. Previous literature states that exogenous melatonin and scheduled feeding accelerated re-entrainment of the SCN-driven general activity after a 6 h of phase advance of the light-dark cycle in rats [
21]. Males exposed to 8 h advanced jetlag conditions, re-entrain faster when treated with melatonin [
22]. A study done on mice reports locomotor activity rhythms and clock protein levels in the SCN of melatonin-deficient (C57BL/6J) and melatonin-proficient (C3H/HeN) mice, as well as in melatonin-proficient (C3H/HeN) mice with targeted deletion of the MT1, MT2, or both receptors exposed to phase delays or phase advances of the light/dark cycle. Endogenous melatonin signal facilitates re-entrainment of the circadian system faster compared to mice which are deficient in melatonin or its receptors [
23]. This information further support that WA may act similar to melatonin, fasting the re-entrainment rate in jetlag exposed mice.
Jetlag or shift work disrupts the normal physiology of mice by altering their oxidative levels, resulting in a low energy level and a decline in their willingness to be active.
Figure 4 depicts how a reduction in movement and an elevation in feeding, which provides a sense of pleasure during stressful situations, leads to weight gain, and WA administration effectively normalizes these detrimental ramifications. The WA-treated group also exhibited faster entrainment, which could be an indirect result of the
PER1 gene upregulation. The
PER1 gene has been proven to play a role in entrainment, and in our prior experiment, we discovered that the WA-treated group had an elevation in
PER1 gene expression, which may enhance the rate of entrainment [
24]. Stress can also impair memory retention, as evidenced by the findings of the Barnes and Y mazes. Compared to all other CJL exposed groups showing less locomotion in the Y maze which could be attributable to stress, WA-treated mice demonstrated increased movement in the same (
Figure 5). The WA-treated group reached the escape hole faster during the Barnes maze test (
Figure 7), possibly because the antioxidant enzyme system fared better, enhancing neuronal function, memory formation, and behavioral performance. Due to its ability to stimulate the neuronal formation and plasticity WA has been validated to reduce neurodegeneration, reconstruct neural networks, and enhance memory retention [
25,
26]. It has been substantiated that WA treatment increases the
BDNF gene expression, an essential factor in neuronal growth and plasticity, resulting in better memory. It has also been reported that
BDNF plays a role in entrainment, as evidenced by in vivo studies with heterozygous
BDNF mutant mice that exhibit impaired light responses [
27] as well as in vitro experiments revealing that this neurotrophin can increase NMDA-induced phase shifts in suprachiasmatic neurons [
28,
29]. In addition to WA’s direct effect on circadian synchronization or jetlag stabilization, these evidence supports our results in a way that also suggests WA can indirectly affect circadian synchronization or jetlag stabilization through behavioral changes and neuronal modifications.
Approximately 20% of the world’s working population engages in shift work in today’s paradigms, making it the norm rather than the exception. Therefore, in order to ameliorate the negative effects of shift work, chronotherapeutics would prove to be beneficial. Our experimental results show that WA-treated mice demonstrated improved locomotor activity with faster synchronization of their phase angle and enhanced cognition in both short-term and long-term memory after chronic jetlag exposure. These behavioral results were further supported by the effective upregulation of the PER1 and BDNF genes in the hippocampus and SCN areas of the brain as well as the restoration of normal body weight in the WA-treated group. Moreover, the increased efficiency of the antioxidant enzyme system in WA-treated mice indirectly relates learning and memory to stress since more stress indicates poorer memory and the SOD and CAT were found to stabilize elevated reactive oxygen radicles in the hippocampus, thereby helping memory retention.
In summary, with its dual benefits and ease of administration, WA, and by extension, Withania extract, would make a valuable addition to the arsenal of agents that can help to combat the negative effects of shift work.