Resting State Electroencephalographic Gamma Activity Is Associated With Circadian Preference in Patients With Depression
Article information
Abstract
Objective
This study aimed to investigate whether there is a relationship between an individual’s circadian preference and differences in quantitative electroencephalography (QEEG) during the daytime, specifically in patients with mood disorders.
Methods
The participants with major depressive disorder, bipolar I disorder, and bipolar II disorder, who had experienced major depressive episodes, were categorized into two or three groups based on their morningness-eveningness preference using the Korean version of the Composite Scale of Morningness (KCSM). The study examined the power of different QEEG bands, such as alpha, beta, theta, delta, and gamma, in the frontal cortex of these patients.
Results
The findings indicated that individuals with eveningness preference had significantly lower power in the high gamma band of the right prefrontal cortex compared to those with morningness preference. The average of high gamma powers in the total frontal cortex was also found to be different between the groups. Furthermore, the study revealed a significant association between K-CSM scores and high gamma power, as well as a history of suicide attempts.
Conclusion
These results suggest that QEEG may serve as a potential biomarker for circadian preference and suicidality in patients with mood disorders.
INTRODUCTION
Circadian rhythms refer to the natural 24-hour cycles that regulate physiological and behavioral processes, including sleep-wake patterns. Morningness and eveningness are two distinct circadian preferences that reflect individual differences in the timing of these cycles [1]. Morningness is characterized by a preference for earlier sleep and wake times, while eveningness is characterized by a preference for later sleep and wake times. These circadian preferences have been associated with differences in cognitive performance, mood, and overall well-being [1-3].
Electroencephalography (EEG) is a non-invasive technique that records electrical activity of the brain through electrodes placed on the scalp. Quantitative electroencephalography (QEEG) is a method of analyzing EEG data that provides a more detailed assessment of the brain’s electrical activity [4]. QEEG has been used to study various aspects of brain function and dysfunction, including sleep disorders. QEEG can provide a more objective measure of circadian preference compared to self-reported questionnaires, which may be subject to bias or variability. QEEG can also provide insight into the underlying neural mechanisms that may contribute to differences in circadian preference [5].
Some investigators examined the difference in quantitative sleep electroencephalograms according to circadian preference in healthy participants. They found that morningness preference (MP) had more spectral power in low sigma (12–14 Hz) compared with eveningness preference (EP) and the decay rate of slowwave activity (1–5 Hz) tended to be faster in MP compared with EP [5]. In another study, individuals with MP presented a steeper decrease of high sigma (14–16 Hz) and beta (16–24 Hz) bands across the night in centro-parietal region than those with EP [6]. Furthermore, individuals with MP reported a higher subjective sleep quality than those with EP. In addition, delta (0.5–3.5 Hz) band declined monotonically across the first 4 NREM/REM cycles in MP while no decrement was observed over the first 2 cycles in EP [7].
However, most studies regarding circadian preference and band powers were limited to comparing sleep EEG. Until now, few studies have compared band powers in EEG according to circadian preference during daytime. In this study, we divided into two (MP and EP) or three groups (MP, intermediate preference [IP], and EP) according to circadian preference to compare the power of alpha, beta, theta, delta, and gamma band. Through this comparison, we will determine whether it is possible to play a role as a biomarker by identifying whether there are characteristic findings of QEEG according to the circadian preference.
METHODS
Subjects and study design
The study is a retrospective analysis of medical records of inpatients with mood disorders, namely major depressive disorder (MDD), bipolar I disorder, and bipolar II disorder, who experienced major depressive episodes as per the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria [8]. The study enrolled 65 patients with information on QEEG and circadian preference using the Korean version of the Composite Scale of Morningness (K-CSM) [9]. Patients with neurological disorders or a history of head trauma, brain surgery, cerebrovascular accidents, or hemorrhage were excluded. The patients were divided into two or three groups based on their circadian preferences: those with MP (n=5), IP (n=31), and EP (n=34); or those with MP-IP (n=31) and EP (n=34). The study compared the band power of EEG and scores on scales such as Hamilton Depression Rating Scale (HAMD) [10] and Hamilton Anxiety Rating Scale (HAMA) [11] between the different groups. The study protocol was approved by the Inje University Ilsan Paik Hospital Ethics Committee (IRB 2022-02-038). Informed consent was waived because of the retrospective nature of the study.
QEEG procedure
E-prime software (Psychology Software Tools, Pittsburgh, PA, USA) was used to synchronize the start of the QEEG recordings and stimulation presentation. QEEG data were acquired with an extended 10–20 deployment method using a Neuroscan SynAmps amplifier (Compumedics USA; El Paso, TX, USA) with 64 Ag-AgCl electrodes on a Quick-Cap. The protocols have been described previously [12]. The spectral power analysis was conducted: delta (1.0–4.0 Hz), theta (4.0–8.0 Hz), alpha (8.0–12.0 Hz), beta (12.0–25.0 Hz), gamma (25.0–40.0 Hz), and high gamma (>40 Hz) bands.
Statistical analyses
Based on circadian preference, a total of 65 patients were classified into two or three groups. The Kolmogorov–Smirnov test was used to check whether the clinical variables were normally distributed. The t-test or Mann–Whitney U test, analysis of variance (ANOVA) or Kruscal-Wallis test, and chi-square test or Fisher’s exact test were used to perform group comparisons according to the properties of variables. Multiple linear regression was used to identify the association between EEG band powers and circadian preference. All tests were two-tailed, and statistical significance was set at p<0.05. Statistical analyses were conducted using the SPSS software package (version 25; IBM Corp., Armonk, NY, USA) and the SALT 2.5 software package (Istech Inc., Goyang, Republic of Korea).
RESULTS
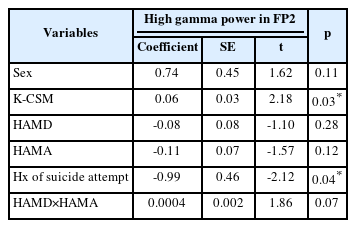
In this study, the demographic and clinical variables were compared among three groups (MP, IP, and EP) in patients with depression. Table 1 showed that there was no significant difference in age, sex, history of suicide attempt, HAMD score, and HAMA score among the three groups. In Table 2, the mean age of patients in the MP-IP group was significantly higher than in the EP group. High gamma power in right prefrontal cortex (FP2) was significantly higher in the MP-IP group than in the EP group, and the average of high gamma powers in frontal region tended to be higher in the MP-IP group compared with the EP group although adjusted p-values were not significant. However, the difference in the total scores of HAMD and HAMA between the groups was not significant. Table 3 revealed that high gamma power in FP2 was positively associated with K-CSM scores, suggesting that as high gamma power increased, the preference for morningness increased, while a history of suicide attempt was negatively associated with high gamma power in FP2. These findings suggest that there may be a relationship between circadian preference, high gamma power in the prefrontal cortex, and suicidal behavior in patients with depression.
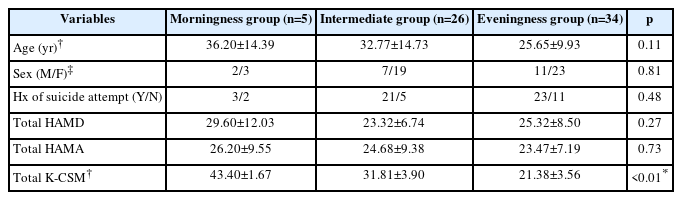
Comparison of demographic and clinical variables between morningness, intermediate, and eveningness preference groups in patients with depression
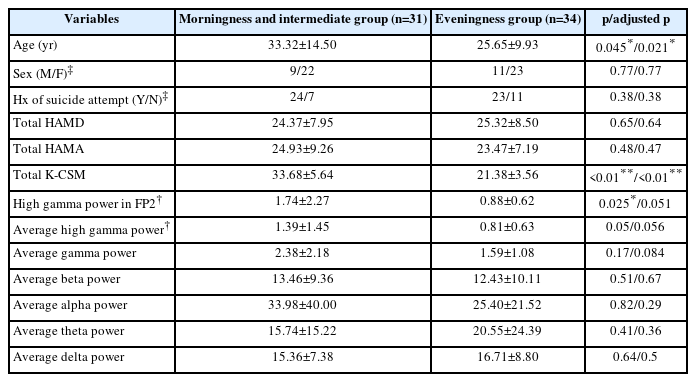
Comparison of demographic and clinical variables between morningness, intermediate, and eveningness preference groups in patients with depression
DISCUSSION
The objective of this study was to explore the relationship between chronotype, clinical features, and QEEG band powers among hospitalized patients with depression. The results showed that the group with EP had lower high gamma power in the FP2 area compared to the groups with MP and IP. Although the average of high gamma powers in the frontal region tended to be lower in the group with EP, this result was not statistically significant. Furthermore, the study found a significant association between K-CSM scores (a measure of chronotype) and high gamma power in FP2, as well as a history of suicide attempts, as determined by multiple linear regression analysis.
The results of this study regarding the link between EP and a history of suicide attempts are consistent with prior research. Some studies have found a significant association between EP and higher levels of depressive symptoms, including suicidality [13,14]. A meta-analysis has also shown that EP is linked to more severe mood symptoms [3]. Furthermore, a study has revealed that individuals in the EP group had higher impulsivity scores than those in the morning and intermediate chronotype groups, and the largest proportion of violent suicide attempters were those with EP [15]. Additionally, there is a higher prevalence of intentional self-harm in individuals with bipolar disorder and EP [16]. However, there have been some conflicting findings, as scores on circadian preference were not correlated with reports of past suicidal ideation or attempts in university students [17], and bipolar patients with a history of suicide attempts had more MP than those without a history of suicide attempts [18]. Thus, the relationship between suicide attempts and chronotype remains controversial, although most studies suggest that EP should be taken into consideration in individuals who may be at risk for suicidal thoughts and behaviors in psychiatric disorders [19].
The observation of decreased high gamma power in the right prefrontal cortex (FP2) of patients with EP is consistent with previous research that has linked EP to abnormal frontal functions [20]. Specifically, some studies have shown that both depressed mood and EP are individually associated with subjective cognitive complaints in individuals with MDD [21]. Furthermore, a study by Hasler et al. [22] has suggested that changes in reward-related brain function could be responsible for the association between EP and alcohol use problems. These findings suggest that alterations in neural activity linked to EP may increase the risk of developing depression. Additionally, prefrontal dysfunction is a well-known characteristic of depression and suicidal tendencies [23]. The FP2 electrode placement refers to the right prefrontal cortex, a brain region involved in executive functions such as decision-making, working memory, and attention [24]. Structural changes in the prefrontal cortex have been associated with various psychiatric disorders, including depression and anxiety [25]. Furthermore, patients with MDD who have attempted suicide have reduced functional connectivity between the bilateral amygdala and prefrontal cortex when compared to individuals with MDD without a history of suicide attempts and healthy controls [26].
Gamma power is believed to enhance effective communication between different regions of the brain and is associated with attention, learning, and memory retrieval [27,28]. The circadian system appears to regulate the frequency of gamma burst activity within cortical and limbic brain regions [29]. Furthermore, gamma power in the prefrontal cortex has been identified as a reliable indicator of MDD, as this brain region plays a significant role in mood and emotional regulation [30]. Numerous studies have reported elevated gamma powers in individuals with MDD [31]. However, one study found that individuals who had attempted suicide had higher gamma power levels than those who had not [32]. Additionally, gamma power in different brain regions varied significantly between patients with MDD and those with bipolar disorder [33,34]. Therefore, the interpretation of gamma power is complex and requires careful consideration.
The present investigation has certain constraints that require attention. Firstly, the number of participants in the MP group was comparatively small, and this could have constrained our ability to observe significant dissimilarities between groups. Secondly, the retrospective design of our study precludes us from establishing a causal relationship between chronotype and clinical traits. Lastly, we did not regulate for potential confounding factors such as medication use, which may have influenced our findings. Additionally, our study involved patients with both bipolar depression and MDD. Thus, this could affect the results.
To summarize, our results indicate that EP is linked to increased levels of depressive symptoms and changes in brain activity among patients with depression. However, larger studies with longer-term observation periods are necessary to gain a more comprehensive understanding of the connection between chronotype and depression, as well as to investigate the neural mechanisms that underlie this association.
Notes
Funding Statement
None
The author has no potential conflicts of interest to disclose.
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available due to privacy or ethical restrictions but are available from the corresponding author on reasonable request.