Impact of a Single Episode of Different Intensity of White Light Pulse at Night on Masking Response in the Diurnal Mammal, Funambulus pennantii
Article information
Abstract
Objective
Light affects mammalian circadian rhythms through entrainment and masking. Photic masking increases locomotor activity in diurnal species (positive masking) while reducing activity in nocturnal species (negative masking). Fewer studies have investigated masking in diurnal rodents. We investigated the masking response in a diurnal rodent, Funambulus pennantii when subjected to white light pulses of different intensities at night between zeitgeber time (ZT) 12 and ZT 15.
Methods
Adult male squirrels, F. pennantii (n=10/group, three groups) were placed in individual cages with running wheels under standard 12:12 h light-dark (LD) conditions. Recording of the locomotor activity rhythm was carried out using a ClockLab setup. Following a week of stable entrainment to the LD cycle, the animals of each group were given a sham exposure. After 10 days, animals of group I, II, and III were exposed to 3-h white light pulses of 100 lux, 10 lux, and 1 lux, respectively, between ZT 12 and ZT 15. Following the exposure, the animals were allowed to run undisturbed for 7 days.
Results
Compared to the shamexposed group, exposure to 3-h 1 lux, 10 lux, and 100 lux artificial light pulses at night caused positive masking, stimulating wheel running in an intensity-dependent manner. Additionally, nighttime light pulses of 100 lux and 10 lux reduced onset accuracy, reduced the amplitude of the rhythm, and also altered the phase angle relationship to light.
Conclusion
The positive masking caused by light exposure at night in diurnal squirrels is intensity-dependent up to at least 100 lux and is associated with a dampening of circadian rhythms.
INTRODUCTION
The hypothalamic suprachiasmatic nuclei (SCN) generate an internal neurogenetic rhythm in mammals with a periodicity of approximately 24 h. These rhythms are synchronized or entrained by external light-dark cycles through photosensitive retinal ganglionic cells via the retinohypothalamic tract. One of the ways in which the external manifestation of the circadian clock may be observed is locomotor activity. Based on when locomotor activity occurs, organisms are categorized into day-active (diurnal), nightactive (nocturnal), or active during twilight hours (crepuscular) [1]. Additionally, some animals are cathemeral, exhibiting activity throughout 24 h and may be considered arrhythmic [1]. A set of physiological, anatomical, and behavioral adaptations further restrict an organism’s activity pattern to a clement spatio-temporal niche [2,3].
Photic input acts as a strong cue in the entrainment of the endogenous clock. Besides this, photic input may also cause masking; in which the duration of the active period may be increased or sharply reduced [4,5]. Entrainment and masking enable the organism to align its behavior according to the external environment while retaining the ability to respond immediately to an unpredictable or fluctuating environment [6]. The (endogenous) photic entrainment of the circadian clock is quite similar in diurnal and nocturnal rodents whereas the (external) response generated by masking is very different [7-9]. Generally, light increases the activity of a diurnal organism, referred to as positive masking, while suppressing it in nocturnal animals (negative masking) [4,10,11].
Masking also depends on the genetic components of the circadian rhythm and the duration of light exposure. For example, a Clock mutant nocturnal mouse exhibited lesser masking with a 1-h light pulse than wild-type mice. Over a longer duration of exposure to the light pulse (3 h), the mutant, however, exhibited a stronger masking response [12]. Masking and entrainment may be complementary ways to enable the best response to the spatiotemporal niche that the organism occupies.
In the contemporary context, artificial light at night (ALAN) may disrupt entrainment or cause unwanted masking. This is associated with a detrimental impact on human health, as well as disruptions in the physiology and lifestyle of wildlife [13,14]. The brightness of light at night is correlated with obesity and metabolic disruption [15]; the incidence of breast [16], colorectal [17], and prostate cancer [18]; reduced melatonin secretion [19]; and disrupted sleep with impaired sleep quality [20,21].
Light at night can also disrupt predator-prey relationships, and reduce success/survival for the predator/prey. It has been shown that ALAN reduces anxiety and neophobia in nocturnal laboratory rodents [22-24], which would have reduced predator avoidance and subsequently their survival in the wild. ALAN may also induce activity at night in diurnal mammals, increasing the chances of their exposure to nocturnal predators. By falsely signaling long days or masking short day signals, light at night can negatively impact seasonal reproduction, leading to reduced availability of predators and prey, further causing ecological imbalance [25,26]. There have been relatively fewer studies on the effect of ALAN in diurnal mammals. Therefore, we studied the locomotor activity response in diurnal squirrels, Funambulus pennantii, to light pulses at night of different intensities.
METHODS
The study used adult male Indian palm diurnal squirrels (F. pennantii) that were of the same age, weighing 120±5 g and measuring 30±2 cm in total length [27]. Squirrels were obtained from the agricultural field of Banaras Hindu University, Varanasi, Uttar Pradesh, India (longitude: 83°1'E; latitude: 25°18'N). Individual squirrels were kept on a 12:12 h light-dark (LD) in clear polysulfone cages measuring 43 cm×27 cm×18 cm with a thin coating of rice bran as bedding (light on at 06:00 h) with ad libitum access to food and water. The intensity of light during the 12:12 h LD cycle was maintained at 250 lux during the photophase and 0 lux during the dark phase. Animals were initially acclimated to the laboratory condition for a month before experimentation. Experimental procedures were conducted in accordance with the guidelines of the Institutional Animal Ethical Committee of the University with its approval (No. F.Sc./88/IAEC/2016-17/1400), and the Guidelines of the Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA) of the Government of India and in conformity with international ethical standards [28].
Experimental design
Animals of all three groups (n=10/group) were housed individually and transferred to separate experimental chambers (light and soundproof) having standard 12:12 h LD condition and introduced to individual cages equipped with running wheels (40.64 cm×50.80 cm×20.96 cm). After a week of stable wheel running (to allow for complete acclimation to running wheels and LD conditions of the chronocubicle), the squirrels of three different groups, i.e., group I, group II, and group III, were given a sham exposure at 0 lux (Supplementary Figure 1). Ten days later, they were exposed to 100 lux, 10 lux, and 1 lux of 3-h single white light pulse at night, respectively, between zeitgeber time (ZT) 12 and ZT15, i.e., from 18:00 to 21:00. These intensities were chosen to represent exposures decreasing by orders of magnitude starting from 100 lux, the approximate level of lighting at the center of a road lit by street lamps in the vicinity of the study area at night. This approximated exposure to ALAN for squirrels dwelling near humans. The procedures were performed with diffuse light from 7 W LED lamps (Philips, Gurgaon, India) dimmed using dimmer switches and butter paper to the required intensity at cage level (Hotek HD2102 Photometer probe, Hotek Technologies, Inc., Tacoma , WA, USA and C-700 Spectromaster, Sekonic Corporation, Tokyo, Japan). Following the exposure, the animals were allowed to run undisturbed for 7 days with maintenance as before.
Wheel revolution data were collected and transferred to a separate computer system for further analysis using a ClockLab (version 6.1.05, Coulbourn Instruments, Whitehall, PA, USA) monitoring system with bin size set at 6 min. An ACT-553 7-channel breakout box and a 56-channel interface (CL-300) were used to record the wheel-running activity rhythm.
Double-plotted actograms were generated and chi-square periodogram, average activity profile, total activity (wheel revolution/day), acrophase (peak of circadian rhythm), alpha (activity duration, α), amplitude, activity onset, the accuracy of onset, and phase angle difference (Ψ) were analyzed. The difference in the onset/offset of activity and onset/offset of the stimulus (lights on/off), respectively were used to compute the phase angle difference. The time interval between successive onsets and offsets of activity represents the alpha. Accuracy is defined as the inverse of the standard deviation in the mean of daily phase angle difference, calculated from the difference between daily onset of activity and the onset of light. Accuracy is an indirect measure of the performance of, and strong coupling between, SCN neurons [29]. The total number of wheel revolutions 7 days prior to the day of exposure and 7 days after exposure was exported to Microsoft Excel 2016. Average daily onsets for the duration of the experiment were plotted.
Statistical analyses
All data were represented as mean±standard error of the mean (SEM). Statistical analyses were performed using Microsoft Excel and GraphPad Prism version 8 (GraphPad Software, Boston, MA, USA). Student’s t-test and one-way analysis of variance (ANOVA) with Tukey’s honestly significant difference (HSD) as a post-hoc test was used to compare the difference between the control and light-pulse groups. Differences were considered significant if p<0.05.
RESULTS
Squirrels exhibited robust circadian rhythm in voluntary wheelrunning locomotor activity. The initial periodicity of the entrained rhythm was 24.0 h, corresponding to the 12:12 h LD cycle (chisquare periodogram).
Effect of 3-h exposure to different intensity of light pulse at night
Effects on circadian locomotor activity rhythm and total activity
White light pulses of 3 h at 100 lux, and 10 lux at night from ZT 12 to ZT 15 caused a transient increase (p<0.001, one-way ANOVA, F(120, 3)=35.64) in locomotor activity during the pulse period, thus causing positive masking, compared to the when no light pulse was administered (Figure 1A-C, Figure 2, and Supplementary Figure 1). This increase in activity was dependent on the intensity of light exposure (Figure 2B, D, F), Spearman rho=0.756. Tukey’s HSD post-hoc test revealed that 1 lux light did not cause a significant change in activity. The entrained period did not change significantly due to any of the light pulses (Figure 1D, E, F). There were no changes in these parameters in the shamexposed group (Supplementary Figure 1).
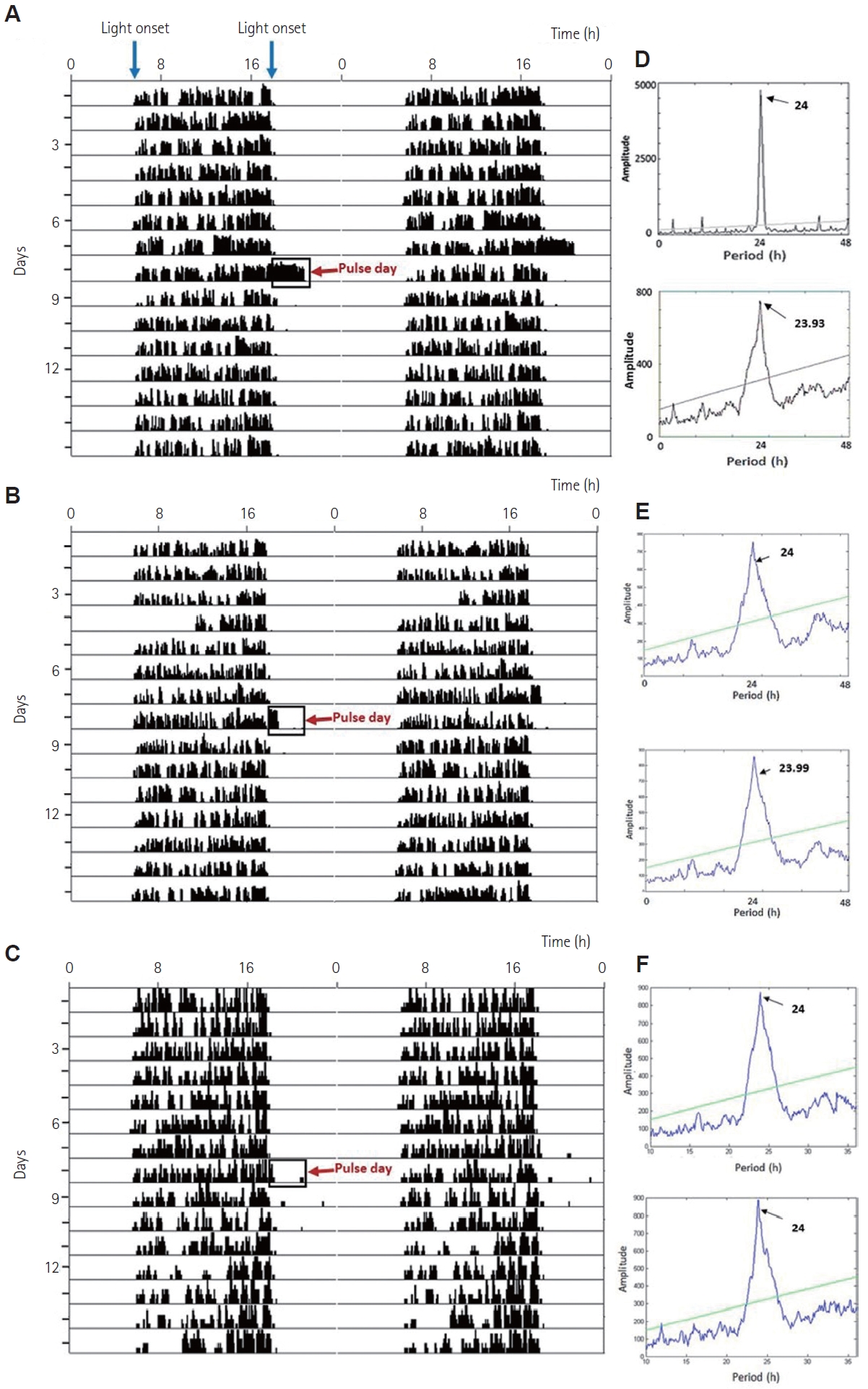
The circadian rhythm in voluntary wheel-running activity in squirrels. Shown here are representative double-plotted actograms before and after a 3-h 100 lux (A), 10 lux (B), and 1 lux (C) light pulse at night (indicated by a clear box and a red arrow) and chi-square periodogram analysis before and after pulse day (D, E, and F, respectively), indicating periodicity and amplitude of rhythm.

Representative figures showing the average pattern of wheel-running activity (counts/min) with time in hours. Cumulative average data are shown for 7 days during 12:12 h light-dark cycke when no light pulse was administered (A, C, and E; pre-exposure) and during the single day of 3-h light pulse of 100 lux, 10 lux, and 1 lux intensity at night between ZT 12 and ZT 15 (B, D, and F; experimental). White and black bars indicate the duration of the light and dark phases, respectively. The onset of the light pulse at ZT 12 is indicated by the dotted line. Sham-exposed controls exposed to 0 lux between ZT 12 and ZT 15 are also represented (G, pre-sham exposure; H, one-day post-sham exposure).
Total activity in terms of wheel revolution showed a significant increase in activity after exposure to 100 lux- and 10 lux-light pulses, groups, but not the 1 lux light pulse, compared to the duration before exposure (p<0.01, p<0.05, and p>0.05, respectively) (Figure 3).

Total activity before and after white light pulse at night. Significant increase in total activity measured in wheel revolution per day (y-axis) in response to the exposure of 3-h white light of 100 lux, 10 lux, and 1 lux intensity, respectively at night between ZT 12 and ZT 15. Data are represented as mean±SEM (n=10/ group). *p<0.05; **p<0.01.
Effects on the amplitude, accuracy of onset, and phase angle relationship
Squirrels exhibited a reduction in the amplitude of the circadian rhythm in wheel running with 100-lux and 10-lux light pulses, but not when exposed to 1 lux pulses or sham exposure at night (F(32, 3) 12.89, p<0.01, one-way ANOVA with Tukey’s HSD post-hoc test) (Figure 4D). A reduction in the phase angle relationship with the light onset was seen in squirrels exposed to 100 lux and 10 lux light (Figure 4C). One lux light pulses and sham exposures (0 lux) did not change the phase angle relationship (Figure 4C). Activity onset was found to be delayed in squirrels exposed to 100 lux and 10 lux pulse at night (Figure 4A and C), but not in 1-lux exposed and sham-exposed animals.

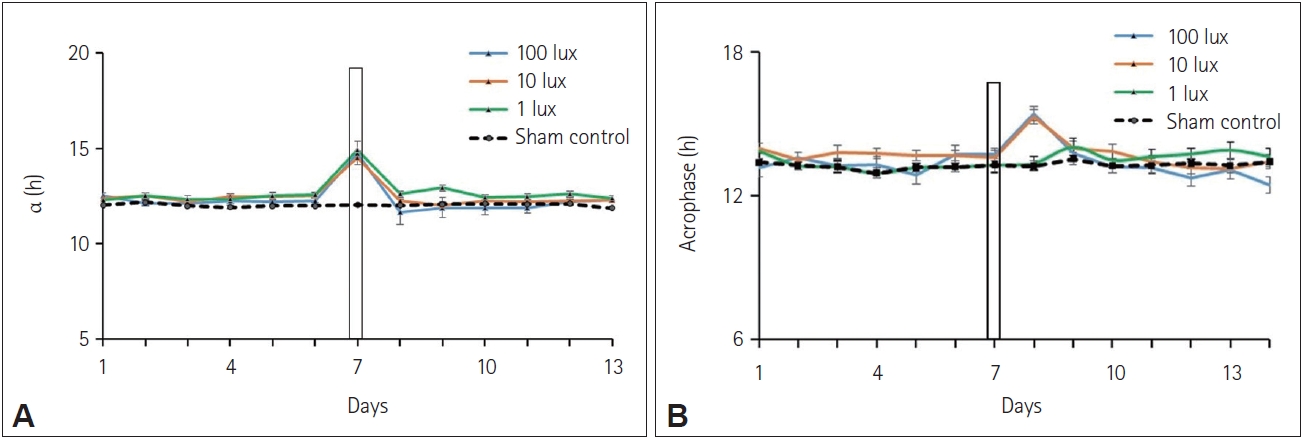
Average daily onsets of activity (A), the accuracy of onset (B), phase angle difference Ψ (phase angle relationship) (C), and change in amplitude (Δ amplitude) in arbitrary units (D); before and after the 3-h 100 lux, 10 lux, and 1 lux white light pulse or sham-exposed control (dashed line) at night between ZT 12 and ZT 15 in Funambulus pennantii. In the average daily onset of activity graph, the day of the light pulse at night is indicated by a vertical bar. Data is represented as mean±SEM (n=10/group). Columns labeled ‘a’ represent data significantly different from columns labeled ‘b’, p<0.01, one-way ANOVA with Tukey’s post-hoc test; *p<0.05
Accuracy of onset was found to be significantly reduced for diurnal squirrels exposed to 100 lux and 10 lux pulse of light at night compared to their respective controls (p<0.05 for both) (Figure 4B). However, 1 lux light pulse at night caused no change in the accuracy of onset compared to the control (Figure 4B), although there was a sizeable reduction in mean accuracy. Similarly, exposure to a 3-h light pulse at night of 100 lux and 10 lux intensity, but not 1 lux, caused a significant change in the phase angle relationship from positive to negative compared to sham control (p<0.05) (Figure 4C).
Effects on alpha and acrophase of the circadian rhythm
Alpha was found to be increased (F(32, 3)=19.5015, p<0.01, oneway ANOVA with Tukey’s post-hoc test) in all three groups exposed to 100 lux, 10 lux, and 1 lux light pulse at night compared to the alpha during the preceding days (Figure 5A). The acrophase of the circadian rhythm for diurnal squirrels kept under 12:12 h LD cycle was found to be at around 1 pm which transiently changed to around 3 pm in squirrels exposed to 100 lux and 10 lux pulse of white light at night. A small non-significant change in the acrophase of the circadian rhythm was also found in squirrels exposed to a 3-h light pulse of 1 lux intensity at night (Figure 5B).
DISCUSSION
We found that the strongly diurnal squirrel, F. pennantii, exhibited positive masking when exposed to light at night of varying intensities. The onset of activity and acrophase were also delayed, adding to evidence that suggests that a single pulse of ALAN may delay the sleep and wake times in diurnal mammals. Thus, a direct and acute effect of ALAN in the form of intensity-dependent positive masking was observed (Figures 1-6) [4]. Additionally, the artificial light pulse at night caused a reduction in the accuracy of onsets, dampening of the amplitude of the rhythm and a reduced phase angle relationship to light significantly with 100-lux and 10-lux pulses. Reduction in these parameters is not prominent with 1-lux light pulses, indicating that the robustness of the rhythm may be affected by light at an intensity of 10 lux or more. 1 lux represents an intensity greater than moonlight, but in our model, it does not appear to cause significant changes in the parameters studied, except for a transient increase in alpha (duration of activity). The report is in accordance with the findings of Bedrosian et al. [30], which suggest that the dampening of circadian rhythm occurs due to the suppression of the activity of the molecular clockwork within SCN. A reduced accuracy, as seen in our study, could be indicative of a loss of coupling between SCN neurons due to exposure to mistimed light at night.
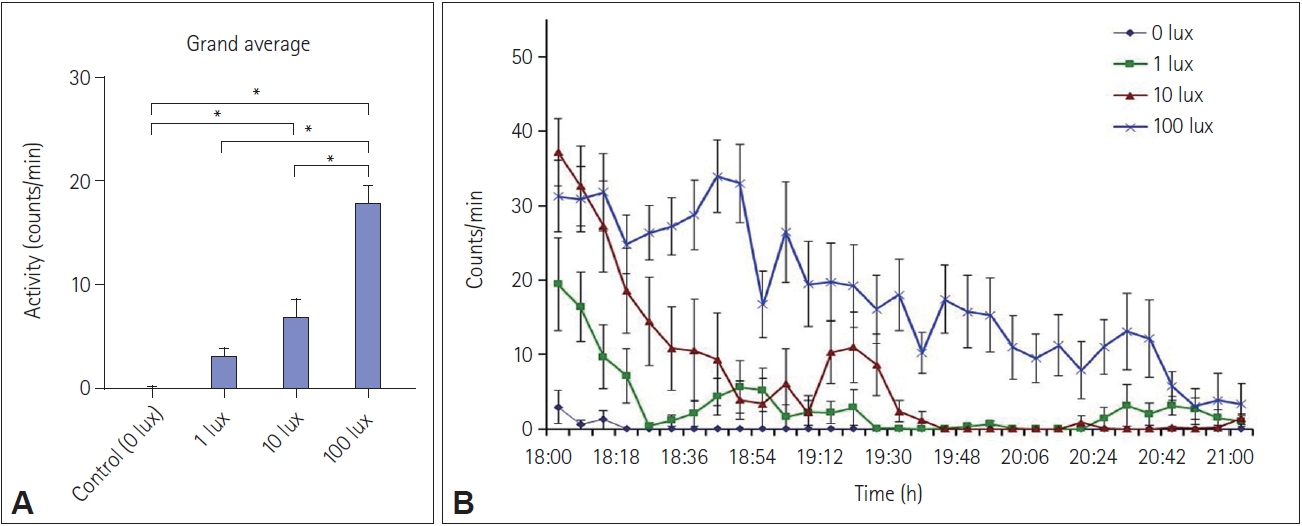
Intensity-dependent increase in wheel running activity during exposure to light (1 lux, 10 lux, and 100 lux) or sham exposure (control, 0 lux). Data are represented as mean±SEM (n=10/group). Overall activity in counts/min (A) and activity in 6-minute bins during the light or sham exposure (18:00–21:00) (B) are shown. *p<0.01, one-way ANOVA with Tukey’s post-hoc test.
While the onset accuracy of the rhythm was reduced by a 1 lux pulse of light, there was no significant difference overall, due to inter-individual variation. This shows that different members of a diurnal population may respond differently to ALAN, and this needs to be taken into account while performing ALAN-related studies. The increase in total average wheel running during light exposure shows the effects of arousal caused in this animal by a single episode of artificial light exposure at night. It is interesting to note that tropical diurnal mammals, which are regularly exposed to daytime sunlight of 20,000 lux intensity and more, respond strongly to a single exposure of 10 lux to 100 lux intensity, which is comparatively dimmer.
Masking is a complex adaptive process and the response elicited by masking is different in day-active and night-active animals, as light is more likely to increase activity in the former, referred to as positive masking, and decrease it in the latter, referred to as negative masking [31-35]. In our case, the activity increased during the pulse period (between ZT12 and ZT15) (Figure 5A and Figure 6), characteristic of positive masking. This is in agreement with findings in Nile grass rats, degus, Mongolian gerbils, and golden spiny mice [9,11,32-34,36-43]. Recently, a comparative study that involved exposure to the same light stimulus to diurnal Nile grass rats and nocturnal mice at the same time of day. This study found that the animals responded differently, i.e., light increased the activity of grass rats and suppressed it in mice [36]. The current findings also agree with other studies that demonstrate duration- and intensity-dependent modulation of activity and the circadian clock by light at night [44,45]. In Figure 6B, it can be seen that exposure to 100 lux of white light is associated with increased activity throughout the duration of the light pulse, but such activity ceases after approximately ZT 13.75 (19:45 clock hours) with exposure to lower intensities of light. A light intensity-dependent arousal mechanism may thus be inferred.
These lines of evidence suggest that in studies with translational value, it may be better to use diurnal, rather than nocturnal models. This would enable better extrapolation of results to predict what may happen in diurnal humans. Mistimed or nearly constant light leads to the desynchronization of biological and physiological rhythm resulting in several negative health consequences. Reports suggest that exposure to light at night, even at very low intensities, strongly inhibits melatonin secretion which may disrupt the overall synchrony of the central with the peripheral clock [46,47]. Apart from melatonin, glucocorticoid secretion is greatly affected by mistimed light exposure by altering the hypothalamic-pituitary-adrenal axis [48]. Studies demonstrated that continuous light exposure can alter activity rhythm and ablate the circadian rhythm of glucocorticoids [15,49].
While we have not explored the mechanistic basis for the phenomenon seen in these squirrels, a probable mechanism is known in the literature. Studies on nocturnal rodents have been used to characterize different light-responsive regions of the brain including evaluation of cFOS expression, revealing that there are considerable differences across brain regions, species, and strains. [50,51]. Fewer studies have examined light-induced cFOS activation in diurnal species and most of these studies focused exclusively on SCN [52-54]. Apart from SCN, light induces cFOS expression in the peri-SCN region of diurnal Nile grass rats and the intergeniculate leaflet of degus [53,54]. Another study demonstrated that cFOS expression in several brain regions including the SCN, ventral subparaventricular zone, intergeniculate leaflet, lateral hypothalamus, olivary pretectal area, and dorsal lateral geniculate, increased in grass rats and decreased in mice [55] after exposure to light at night. These areas might produce acute effects on general activity through pathways extending to the structure that regulates the sleep/wake cycle, as light triggers sleep in nocturnal mammals (e.g. mice) and increases arousal or alertness in diurnal animals (e.g. humans) [31,56-58]. Similarly, in our case where a diurnal rodent was used, the light pulse between ZT12 and ZT15 might be activating the center that is involved in alertness and varying intensity of white light might be triggering different levels of activation. We did not study mechanisms of post-ALAN arousal in this study, which is a limitation of the study.
While the sleep-promoting effects of melatonin are known [59-64], it has been shown that melatonin does not induce a sleep-promoting effect [65,66] in F. pennantii. Another study proved that pinealectomy did not alter general locomotor activity rhythm in Avicanthis niloticus. This suggests that melatonin, or its light-induced suppression, may not be contributing significantly to the phenomenon observed.
Kumar et al. [65] demonstrated that squirrels exposed to a 3.5:3.5 h cycle exhibited a prominent masking response. Additionally, exposure to light results in a significant increase in activity during the light phase compared to the dark, demonstrating that diurnal squirrels are positively masked by light. Two other studies have shown that the activity rhythm of diurnal rodents is masked by lighting conditions, with activity peaks occurring during the light period of a 3.5 h ultradian cycle [1,36]. This agrees with our study in 12:12 h LD conditions, which better represents real-world conditions in the sub-tropical latitudes to which this squirrel is native.
Exposure to unnatural lighting schedules increases the risk of cancer, sleep, and mood disorders [16,67,68]. Furthermore, nighttime light exposure is associated with metabolic disorders and stress [13,14,15,30,49], as evinced by the measurement of associated biochemical and genetic parameters as well as cortisol. For instance, shift workers who experienced nighttime light illumination are at increased risk of cardiovascular disease and elevated body mass index [69-71]. Low intensities of light at night may inhibit body mass gain by delaying the time of food intake and limiting food access to the dark [15].
Our study clearly demonstrates the need for establishing a variety of diurnal models in the study of photic resetting of the circadian rhythm. The effects of a single pulse of ALAN observed in our study are very different from known effects in nocturnal animals. Detailed comparative studies are needed to elucidate further at molecular and behavioral levels to understand the neuronal circuitry in both groups.
Supplementary Materials
The online-only Data Supplement is available with this article at https://doi.org/10.33069/cim.2023.0015.
The circadian rhythm in voluntary wheel-running activity in squirrels. Shown here is a representative double-plotted actogram before and after a 3-h sham exposure (0 lux) at night on the 8th day, and chisquare periodogram analysis for the entire duration indicating periodicity (h) and amplitude of rhythm (arbitrary units). The sham exposure did not change the parameters studied.
Notes
Funding Statement
This research was supported by the University Grants Commission (UGC), New Delhi (No.6-2/2017/IC) to MS under the UGCISF Joint Research Program.
The authors have no potential conflicts of interest to disclose.
Availability of Data and Material
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Muniyandi Singaravel, Vivek Verma. Data curation: Vivek Verma, Priyoneel Basu. Formal analysis: Vivek Verma, Priyoneel Basu. Funding acquisition: Muniyandi Singaravel. Investigation: all authors. Methodology: Vivek Verma, Priyoneel Basu. Project administration: Muniyandi Singaravel. Resources: Muniyandi Singaravel. Supervision: Muniyandi Singaravel. Validation: Muniyandi Singaravel, Priyoneel Basu. Writing—original draft: Priyoneel Basu, Vivek Verma. Writing—review & editing: all authors.