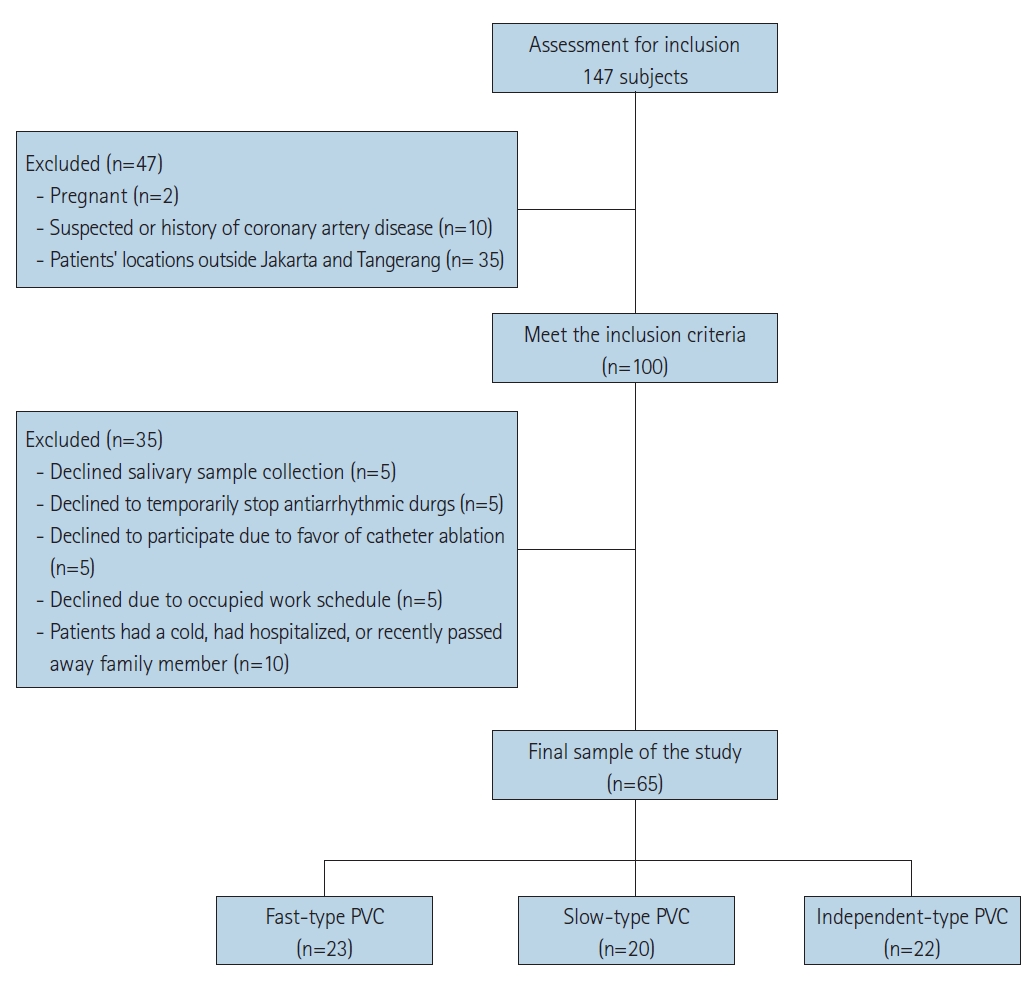
|
 |
- Search
| Chronobiol Med > Volume 5(3); 2023 > Article |
|
Abstract
Objective
Methods
Results
Supplementary Materials
Supplementary┬ĀTable┬Ā1.
NOTES
Conflicts of Interest
Availability of Data and Material
The data will be available for the readers if requested for further PVC circadian rhythm research.
Author Contributions
Conceptualization: all authors. Data curation: Novita Gemalasari Liman, Sunu Budhi Raharjo, Bambang Budi Siswanto. Formal analysis: Novita Gemalasari Liman, Sunu Budhi Raharjo, Joedo Prihartono, Bambang Budi Siswanto. Investigation: Novita Gemalasari Liman, Sunu Budhi Raharjo, Ina Susianti Timan, Bambang Budi Siswanto. Methodology: Novita Gemalasari Liman, Sunu Budhi Raharjo, Ina Susianti Timan, Bambang Budi Siswanto. Project administration: Novita Gemalasari Liman, Sunu Budhi Raharjo, Bambang Budi Siswanto. Resources: all authors. Software: Novita Gemalasari Liman, Sunu Budhi Raharjo, Bambang Budi Siswanto. Supervision: all authors. Validation: all authors. Visualization: all authors. WritingŌĆöoriginal draft: Novita Gemalasari Liman. WritingŌĆöreview & editing: all authors.
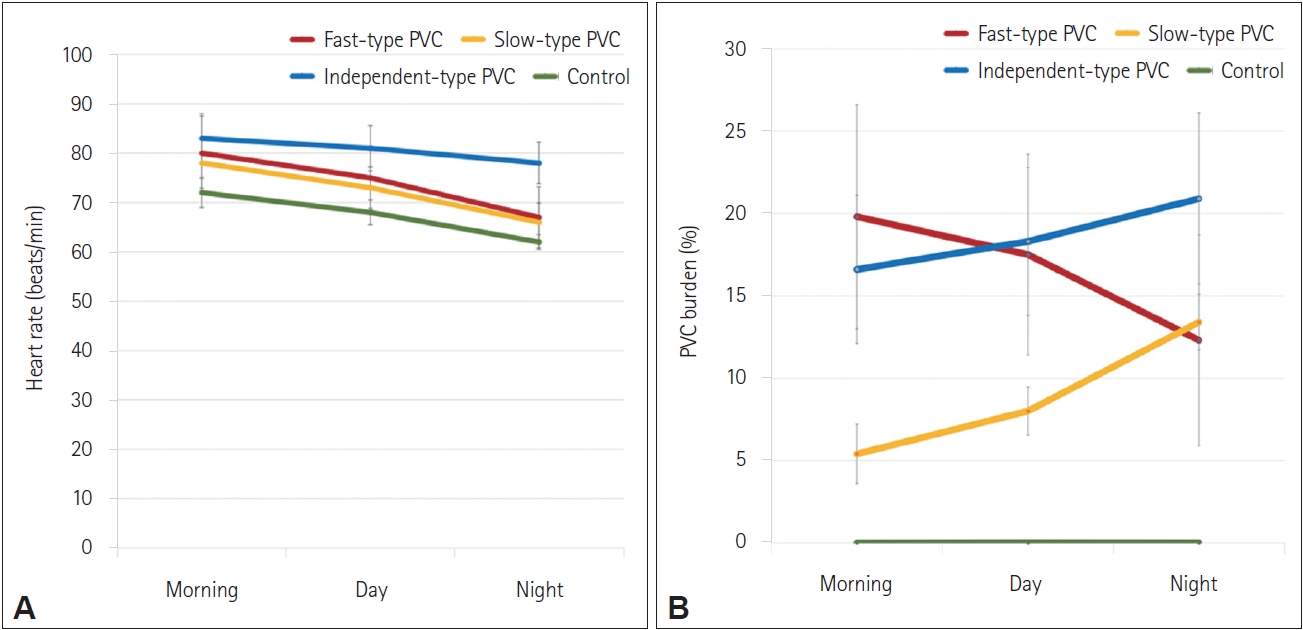
Figure┬Ā2.
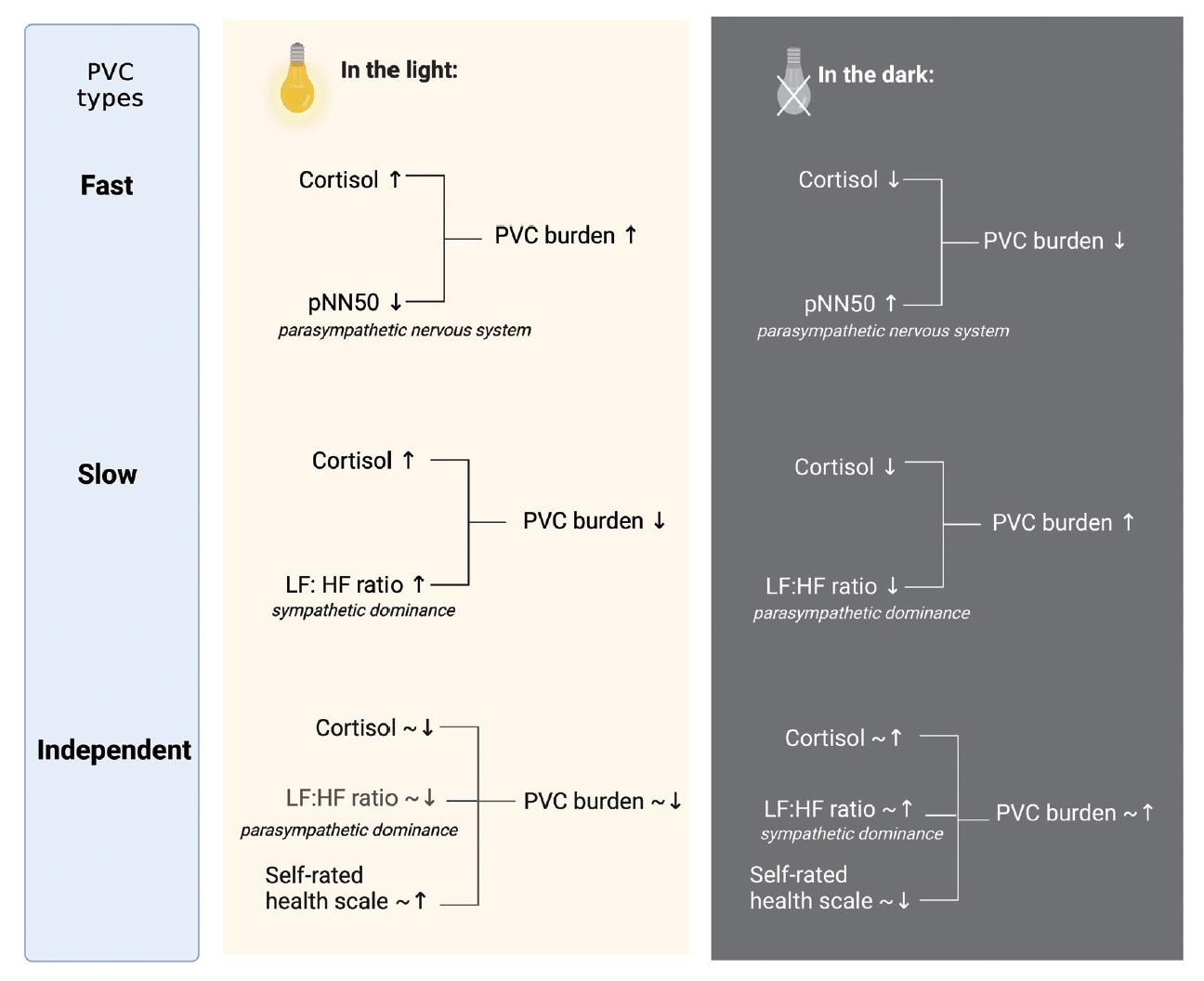
Figure┬Ā3.
Table┬Ā1.
| Characteristic | Fast-type (n=23) | Slow-type (n=20) | Independent-type (n=22) | p | Control (n=5) |
|---|---|---|---|---|---|
| Age (yr) | 51┬▒12 | 44┬▒13 | 45┬▒13 | 0.237* | 40┬▒6 |
| Gender | 0.594ŌĆĀ | ||||
| ŌĆāMale | 4 | 6 | 6 | 2 | |
| ŌĆāFemale | 19 | 14 | 16 | 3 | |
| Marital status | 0.988ŌĆĀ | ||||
| ŌĆāNever married | 2 | 2 | 2 | 1 | |
| ŌĆāMarried | 21 | 18 | 20 | 4 | |
| Education | 0.392ŌĆĀ | ||||
| ŌĆāLess than high school | 16 | 17 | 15 | 2 | |
| ŌĆāHigh school graduate | 7 | 3 | 7 | 3 | |
| Employment status | 0.562ŌĆĀ | ||||
| ŌĆāUnemployed | 14 | 9 | 11 | 2 | |
| ŌĆāEmployed | 9 | 11 | 11 | 3 | |
| Alcohol consumption | 0.791ŌĆĀ | ||||
| ŌĆāNo | 21 | 20 | 22 | 5 | |
| ŌĆāOccasionally | 2 | - | - | - | |
| Dyslipidemia | 0.053ŌĆĀ | ||||
| ŌĆāNo | 14 | 13 | 20 | 4 | |
| ŌĆāYes | 9 | 7 | 2 | 1 | |
| Diabetes | 0.777ŌĆĀ | ||||
| ŌĆāNo | 23 | 20 | 20 | 5 | |
| ŌĆāYes | - | - | 2 | - | |
| Hypertension | 0.002ŌĆĀ | ||||
| ŌĆāNo | 11 | 6 | 18 | 3 | |
| ŌĆāYes | 12 | 14 | 4 | 2 | |
| CCB use | 0.501ŌĆĀ | ||||
| ŌĆāNo | 20 | 17 | 22 | 5 | |
| ŌĆāUse | 3 | 3 | - | - | |
| Beta-blocker use | 0.599ŌĆĀ | ||||
| ŌĆāNo | 8 | 10 | 9 | 3 | |
| ŌĆāYes | 15 | 10 | 13 | 2 | |
| BMI (kg/m2) | 25.6┬▒4.4 | 23.4┬▒2.4 | 24.9┬▒3.9 | 0.167* | 24┬▒4.1 |
| PSS score | 16.1┬▒3.8 | 16.3┬▒5.0 | 17┬▒7 | 0.925 | 16┬▒2.4 |
Table┬Ā2.
| Parameter |
Idiopathic PVC types |
p | Control | ||||
|---|---|---|---|---|---|---|---|
| Fast-type | Slow-type | Independent-type | |||||
| Cortisol (nmol/L) | <0.001*ŌĆĀ | ||||||
| Morning | 18.95 (2.61) | 23.94 (4.12) | 5.21 (0.60) | 10.8 (1.5) | |||
| Day | 32.68 (4.65) | 16.41 (1.86) | 8.49 (1.34) | 20.5 (4.1) | |||
| Evening | 3.67 (0.53) | 5.16 (0.91) | 16.08 (1.82) | 2.6 (0.6) | |||
| Norepinephrine (pg/mL) | <0.001*ŌĆĀŌĆĪ | ||||||
| Morning | 108.33 (23.62) | 20.65 (1.89) | 139.94 (10.48) | 21.9 (3.8) | |||
| Day | 28.65 (6.51) | 160.59 (15.64) | 143.85 (18.54) | 125.6 (11.0) | |||
| Evening | 43.64 (8.11) | 152.48 (20.94) | 111.82 (9.01) | 159.2 (17.7) | |||
| HRV: global activity | |||||||
| SDNN (ms) | <0.001*ŌĆĀ | ||||||
| Morning | 99 (20) | 97 (22) | 59 (13) | 84 (5) | |||
| Day | 136 (22)ŌĆĪ | 136 (32) | 78 (19) | 115 (7) | |||
| Evening | 118 (20)ŌĆĪ | 144 (25) | 89 (22) | <0.001*ŌĆĀ | 104 (26) | ||
| SDANN (ms) | |||||||
| Morning | 80 (16) | 84 (16) | 49 (12) | 66 (8) | |||
| Day | 120 (24) | 131 (28)ŌĆĪ | 71 (17) | 100 (7) | |||
| Evening | 94 (22) | 116 (25)ŌĆĪ | 78 (18) | 78 (6) | |||
| HRV: parasympathetic activity | |||||||
| RMSSD (ms) | <0.001*ŌĆĀ | ||||||
| Morning | 37 (10) | 34 (8) | 23 (6) | 28 (5) | |||
| Day | 42 (8) | 42 (9) | 25 (5) | 34 (6) | |||
| Evening | 48 (10)ŌĆĪ | 57 (13) | 29 (7) | 43 (8) | |||
| pNN50 (%) | <0.001*ŌĆĀ | ||||||
| Morning | 8.06 (1.5) | 7.49 (1.4) | 4.5 (1.2) | 8.3 (1.3) | |||
| Day | 12.03 (2.4) | 10.41 (2.2) | 4.7 (1.9) | 12.4 (2.9) | |||
| Evening | 17.69 (2.1) | 17.96 (2.6) | 4.7 (1.4) | 20.5 (3.8) | |||
| HF (ms) | <0.001*ŌĆĀ | ||||||
| Morning | 0.22 (0.04) | 0.22 (0.08) | 0.13 (0.08) | 0.22 (0.03) | |||
| Day | 0.27 (0.04) | 0.26 (0.10) | 0.14 (0.07) | 0.26 (0.03) | |||
| Evening | 0.33 (0.09) | 0.34 (0.12) | 0.14 (0.07) | 0.34 (0.05) | |||
| HRV: sympathetic activity | |||||||
| LF (ms) | 0.011*ŌĆĀ | ||||||
| Morning | 0.27 (0.09) | 0.26 (0.08) | 0.14 (0.09) | 0.23 (0.06) | |||
| Day | 0.29 (0.05) | 0.29 (0.11) | 0.15 (0.08) | 0.24 (0.06) | |||
| Evening | 0.33 (0.05) | 0.36 (0.19) | 0.16 (0.09) | 0.26 (0,08) | |||
| HRV: sympathovagal balance | |||||||
| LF:HF ratio | 0.050 | ||||||
| Morning | 1.27 (0.28) | 1.29 (0.22) | 1.07 (0.20) | 1.14 (0.36) | |||
| Day | 1.19 (0.25) | 1.24 (0.23) | 1.12 (0.17) | 1.03 (0.27) | |||
| Evening | 1.08 (0.29) | 1.16 (0.30) | 1.19 (0.16) | 0.84 (0.20) | |||
| Self-rated health scale | 0.207 | ||||||
| Morning | 7.89 (1.5) | 7.5 (1.0) | 6.75 (2.3) | 8.2 (0.89) | |||
| Day | 7.67 (1.5) | 7.0 (1.1) | 6.63 (1.9) | 6.6 (0.45) | |||
| Evening | 6.67 (1.7) | 6.25 (1.2) | 6.25 (1.9) | 7.0 (1.73) | |||
p value represents post hoc comparisons between the three PVC types by ANOVA (using a Games-Howell method). HRV, heart rate variability; PVC, premature ventricular contraction; SDNN, standard deviation of normal R-R intervals; SDANN, standard deviation of the averages of normal-to-normal intervals in all 5-minute segments; RMSSD, root mean square of successive R-R interval differences; pNN50, percentage of normal R-R intervals that differ by 50 ms; HF, high frequency; LF, low frequency
Table┬Ā3.
HRV, heart rate variability; PVC, premature ventricular contraction; SDNN, standard deviation of normal R-R intervals; SDANN, standard deviation of the averages of normal-to-normal intervals in all 5-minute segments; RMSSD, root mean square of successive R-R interval differences; pNN50, percentage of normal R-R intervals that differ by 50 ms; HF, high frequency; LF, low frequency
Table┬Ā4.
HRV, heart rate variability; PVC, premature ventricular contraction; SDANN, standard deviation of the averages of normal-to-normal intervals in all 5-minute segments; RMSSD, root mean square of successive R-R interval differences; pNN50, percentage of normal R-R intervals that differ by 50 ms; HF, high frequency; LF, low frequency; N/A, not applicable
REFERENCES
- TOOLS