INTRODUCTION
Sleep is fundamental to human health, with profound implications for mental and physical well-being [
1]. Regular sleep is crucial for maintaining immune function integrity and a homeostatic immune defense against microbial or inflammatory insults. Sleep deprivation may result in deregulated immune responses with increased proinflammatory signaling [
2,
3]. This may contribute to an increased risk of inflammatory chronic conditions, such as cardiometabolic, neoplastic, autoimmune, and neurodegenerative diseases [
4-
6].
Sleep is a physiological process orchestrated by intricate neural networks and hormonal signaling. The immune system helps regulate sleep [
7]. Tumor necrosis factor-alpha (TNF-α), a pivotal proinflammatory cytokine, is a key player in the dynamic relationship between sleep and the immune system. TNF-α’s circadian production is closely tied to the sleep-wake cycle. It peaks during the night and early morning, coinciding with a natural dip in alertness and the sleep propensity. This temporal alignment suggests that TNF-α plays a regulatory role in the circadian regulation of sleep [
8]. Experiments conducted by Besedovsky et al. [
9] revealed that increased TNF-α levels, whether induced by experimental sleep deprivation or other means, increased slowwave sleep, emphasizing the role of TNF-α in sleep regulation. Also, conditions characterized by chronic inflammation, such as rheumatoid arthritis and inflammatory bowel disease, often coincide with sleep disturbances. Dysregulation of TNF-α may contribute to both the inflammatory process and sleep disruptions [
10].
Melatonin, primarily produced by the pineal gland in response to darkness, regulates the sleep-wake cycle and circadian rhythms. Prolonged-release of melatonin (PRM) provide a controlled and sustained release of melatonin over an extended period, aligning with the natural circadian rhythm of melatonin secretion. A study by Wade et al. [
11] suggested that PRM significantly improved sleep quality and morning alertness compared to a placebo and had a favorable safety profile. Melatonin is also known for its antiinflammatory properties, and it modulates immune cell activity and reduces the production of proinflammatory cytokines.
We hypothesize that the consumption of PRM as a first-line defense against insomnia may modulate plasma levels of TNF-α and quality of life (QoL). We also investigated whether the change in sleep quality was associated with the changes in TNF-α levels and QoL.
METHODS
This was a prospective open-label study involving 67 subjects from 6 different hospitals: Pusan National University Yangsan Hospital, Inje University Ilsan Paik Hospital, Gachon University Gil Hospital, Eulji University Hospital, Korea University Ansan Hospital, and Gyeongsang National University Hospital. The selection criteria were patients over the age of 55 years who met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) insomnia criteria. We excluded patients with sleep and mental disorders other than insomnia from the study; however, we included patients with mild depression (<15 points on the Hamilton Depression Rating Scale [HDRS] [
12]). We assessed sleep quality via the Pittsburgh Sleep Quality Index (PSQI) at the baseline, week 4, and week 8 after treatment with 2 mg PRM. Higher scores indicate a worse sleep quality, while values >5 indicate poor quality of sleep [
13].
To assess QoL, we used the WHO-5 Well-Being Index [
14] at baseline and after 4 and 8 weeks of treatment. Answers are provided on a 5-point Likert scale, with higher scores representing a better QoL. To examine the inflammatory state, we measured plasma TNF-α levels by blood sampling before and after 8 weeks of treatment. We employed statistical tests such as repeatedmeasures analysis of variance to investigate the changes in sleep quality and QoL. We used paired t-tests to assess the changes in plasma TNF-α levels before and after 8 weeks of treatment. We analyzed the association between the change in sleep quality and the change in TNF-α level and the association between the change in sleep quality and the change in QoL using Pearson’s correlation coefficients. We utilized Pearson’s correlation coefficients to explore whether baseline status correlated to differences in sleep quality, inflammatory status, and QoL. All tests were two-tailed, and the cut-off p-value for statistical significance was set at p<0.05. We performed all statistical analyses using SPSS software (version 25, IBM Corp., Armonk, NY, USA). The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practices. Written informed consent was obtained from all subjects. The study protocol was approved by the ethics committees of each university hospital (IRB No. 2015AS0064).
RESULTS
Table 1 describes the patient population. The average subject age was 66 years and the average HDRS score was 8.61.
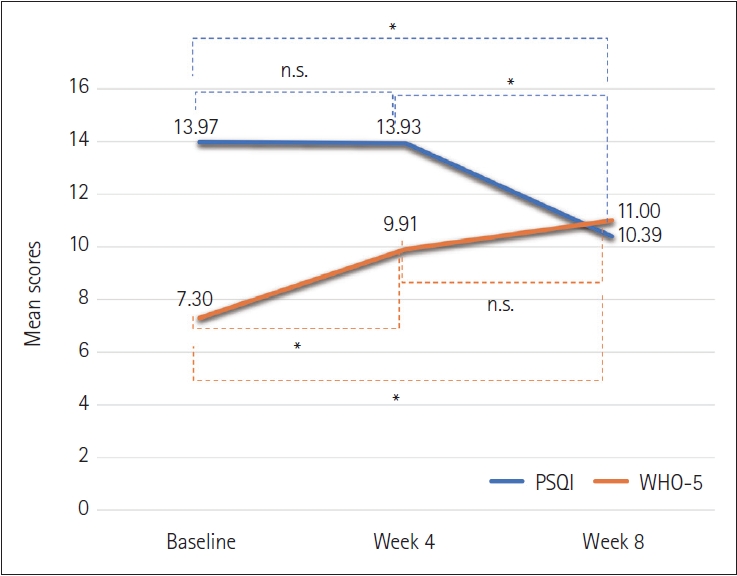
Table 2 shows the mean PSQI score at baseline and weeks 4 and 8. The PSQI score significantly decreased over the study period. Although the average score at week 4 was lower than baseline, this change was not significant (diff=0.05, p=1.00). The week 8 score was lower than the week 4 score. These changes were significant (diff=3.54, p<0.001) (
Figure 1). The mean WHO-5 score improved from 7.30 to 11.0 (p<0.001) during the study period. The increase between baseline and week 4 was significant (diff=-2.61, p<0.001). However, the differences between week 4 and 8 were not significant (diff=-1.09, p=0.14). Mean TNF-α values also nonsignificantly declined from 0.62 to 0.60 over 8 weeks (p=0.28).
There was an association between the change in PSQI and the change in WHO-5 between baseline and week 8 (r=-0.44, p< 0.001). However, there was no association between the change in PSQI and the change in TNF-α between baseline and week 8 (r=0.13, p=0.33).
The improvement of the PSQI over the 8-week study period was correlated with the baseline PSQI score (r=0.40, p=0.001). The decrease in TNF-α levels over the study period was correlated with the baseline TNF-α level (r=0.43, p<0.001).
DISCUSSION
Recent research has illuminated the multifaceted roles of melatonin in human health, including its interaction with TNF-α. Research by Carrillo-Vico et al. [
15] demonstrates that melatonin can reduce TNF-α synthesis in immune cells. Additionally, research by Srinivasan et al. [
16] indicates that melatonin can downregulate TNF-α receptor expression. Our research found a nonsignificant decrease of TNF-α in the plasma after PRM. Intriguingly, the decrease in TNF-α over the study period was correlated to the baseline level of TNF-α. This indicated that melatonin may exert a significant antiinflammatory effect in chronic inflammation or autoimmune diseases involving excessive TNF-α production.
Research by Riemann et al. [
17] suggests that melatonin supplementation can improve sleep-related QoL in individuals with insomnia. This study found that melatonin not only improved objective sleep measures but also subjective assessments of sleep quality, daytime functioning, and emotional well-being. Our data also revealed improvements in sleep quality and QoL, which were significantly associated. We only can report on associations, not cause and effect relationships between sleep and QoL, as multiple factors impact QoL. We found that melatonin improves QoL first and then increases sleep quality. QoL may improve with enhanced daytime functioning, but it may take longer for sleep quality to improve.
There are several limitations to this study. We measured TNF-α concentrations twice: before PRM administration and at a sufficient interval afterward. Since drawing blood is an invasive procedure, we decided against confirming TNF-α blood concentrations during the intermediate phase. Secondly, while several proinflammatory cytokines regulate sleep, TNF-α might be more sensitive to confounding factors than other cytokines. We may have reported different results with other inflammatory molecules. Additionally, several methodological errors, including the duration of sleep deprivation, circadian phase, and the timing of blood sampling, may impact the results. The absence of a placebo group limits our results as well. Finally, our relatively small sample size limits the generalizability of our results. The data presented here must be confirmed with a larger sample size.
Despite these limitations, PRM can be used as an initial treatment to improve sleep parameters and address the associated inflammatory status. The antiinflammatory properties of melatonin and its positive impact on sleep quality and overall wellbeing, make it a valuable potential intervention for individuals with insomnia.