Exercise Timing and Cancer Treatment: Avenues for Chronobiological Research
Article information
Abstract
Emerging evidence suggests that cancer chronotherapy, which involves timing drug administration in accordance with a patient’s circadian/internal time, can improve treatment tolerability and efficacy. However, as cancer, its treatments, and even hospitalization can cause circadian rhythm misalignment, cancer chronotherapy may be attenuated from the start. The adjunctive therapeutic strategy ‘exercise’ not only improves physical function and patient-reported outcomes, but specific effects are determined by the individual’s internal time and it may act as a circadian time cue. Utilizing differentially timed exercise in terms of targeting time-specific homeostatic responses to the activity, accounting for the time of peak performance, and/or targeting potential circadian system effects of exercise could thus potentiate cancer chronotherapy or cancer treatment more generally. Herein, we briefly overview cancer chronotherapy and exercise medicine in oncology, highlight potential benefit of timed exercise in cancer treatment, and discuss research opportunities to assess these potentials benefits.
INTRODUCTION
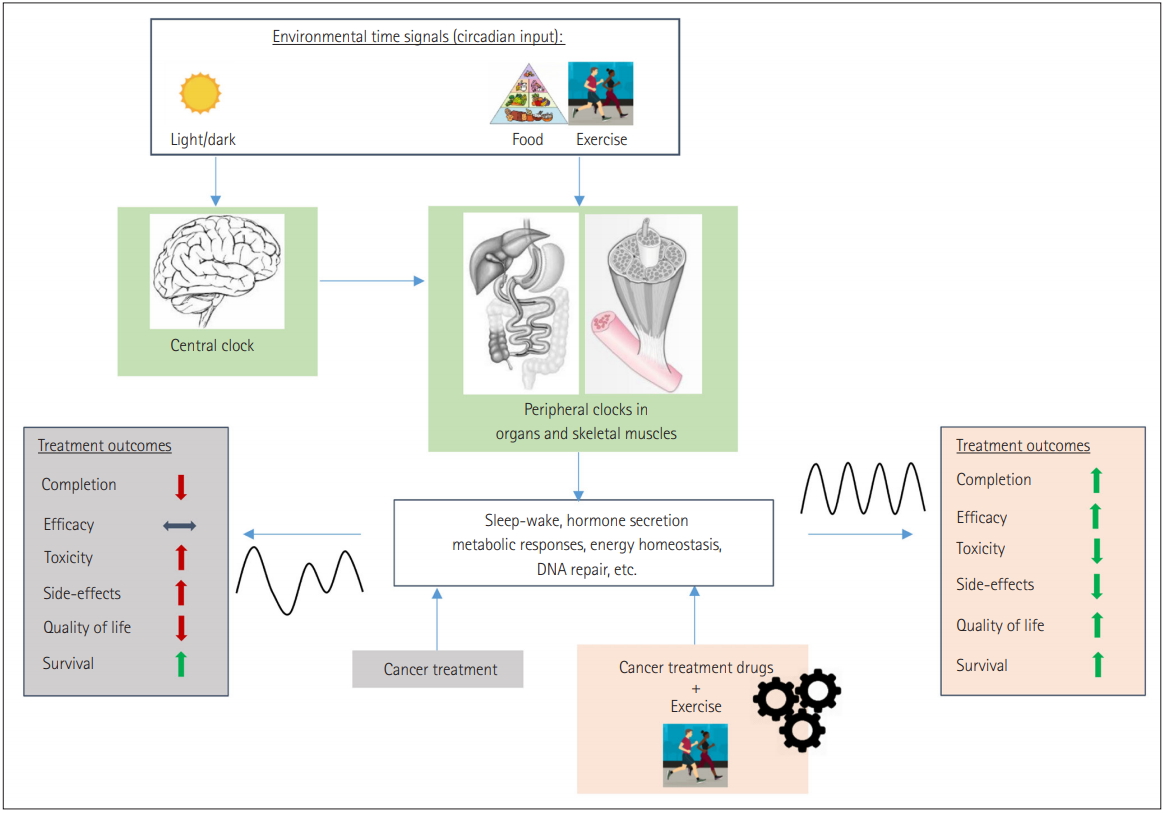
The human circadian timing system temporally organizes myriad physiological parameters across 24 hours (‘circa diem’ from Latin meaning ‘about a day’) from the highs and lows observed in gene expression in cells to the highs and lows in physical and cognitive performance, and much more in between [1,2]. With particular relevance to cancer treatment wherein anticancer drugs can have aggressive, cell damaging, side effects, there are circadian highs and lows in cellular growth, DNA repair, and metabolism [2-5]. Indeed, differentially timed administration of anticancer drugs can elicit differential trade-offs between efficacy and side effects [6,7]. Consideration of circadian timing in anticancer drug administration is commonly referred to as cancer chronotherapy. Of course, cancer, its treatments, and hospitalization may also disrupt the circadian timing system and sleep of the patient [6,8-10]. If treatment efficacy and tolerability depends on the patient’s circadian/internal time, disruptions to circadian timing or misalignment of circadian rhythms should be avoided. The goal must be to optimize circadian alignment in the patient to potentiate timed treatment and attenuate side effects. To this end, timed exercise may represent a valid and valuable, adjunctive, therapeutic strategy. Exercise is already encouraged as part of the treatment process and specified timing may, inter alia, exert chronobiological or diurnal effects that could aid cancer chronotherapy [11-15]. Herein, we briefly overview cancer chronotherapy and exercise medicine in oncology, highlight potential benefits of timed exercise in cancer treatment, and discuss research opportunities to assess these potential benefits.
CANCER CHRONOTHERAPY
Cancer chronotherapy involves specifically timed administration of anticancer drugs according to the individual’s circadian/internal time to improve treatment tolerance and efficacy [6,16,17]. That is to say, there will be an optimum time of day when trade-off between efficacy and side-effects are best based on whether particular circadian rhythms (for instance, cell growth, DNA repair, and metabolism [2-5]) are closer to their peaks or troughs in different tissues. In return for better-timed treatment, better treatment tolerability may lessen circadian disruptive effects of cancer, treatment, and hospitalization [6,8-10]. Furthermore, increased tolerability can facilitate further drug administration [7,16]. Based on experimental models, the chemotherapy tolerability can vary 2- to 10-fold depending on the circadian timing of administration [16]. As of 2010, this pattern exists for at least 40 anticancer drugs; there are likely to be more in the meantime [16]. Evidently, timing can be important. Although the current application of cancer chronotherapy is limited, there have been promising findings from clinical trials. For instance, a meta-analysis of metastatic cancer patients receiving 5-fluorouacil, leucovorin, and oxaliplatin in chronomodulated infusions revealed significantly improved survival (median overall survival: 20.8 months) compared to those receiving conventional infusions (median overall survival: 17.5 months, p-value for difference=0.009) [18].
Clearly, cancer chronotherapies are focused on the internal time of the individual, and rightly so. But as cancer, its treatment, and hospitalization can have detrimental effects on a patient’s sleep and circadian timing system, other factors that improve circadian rhythm alignment or lessen circadian disruption experienced by the patient may provide added benefit when combined with cancer chronotherapy.
EXERCISE MEDICINE IN ONCOLOGY
Exercise is a subset of physical activity that is planned, structured and repetitive with a purpose to improve health [19]. Recently, a consensus report called for increased exercise prescription in oncology and urged healthcare providers to actively promote and provide advice on physical activity, exercise, and appropriate exercise programs for cancer patients and survivors [11]. A body of literature supports the hypothesis that exercise can improve cancer treatment outcomes, quality of life and decrease risk of recurrence in cancer survivors [11]. To date, there have been approximately 700 exercise intervention studies conducted in the cancer survivor population [11,20]. Studies conducted in a mixed population, including cancer patients who were either receiving active treatment or have completed treatment, found physical activity/exercise improved survival among patients with malignant, recurrent glioma [21] and breast cancer [22].
In contrast, and despite growing evidence in support of the benefits of exercise in cancer survivors, few interventions have examined the effect of exercise on anticancer drug treatment tolerance and efficacy. There is promising data from animal studies indicating exercise may improve anticancer drug efficacies. Compared to mice treated with chemotherapy alone, mice treated with exercise plus chemotherapy presented with delayed tumor growth in models of breast [23], melanoma [24], and pancreatic [24] cancers. A similar beneficial effect of exercise was observed to improve tamoxifen treatment in a mouse model of breast cancer [25]. In human studies, inconsistent results are reported: some indicate improvements [21,26-28], others indicate null findings [29-34].
Overall, several studies exploring adjunctive exercise interventions and treatment efficacy outcomes among cancer survivors indicate no adverse effect on treatment efficacy [28,29,33-35].
EXERCISE TIMING AND CANCER TREATMENT
The timing of exercise can affect peak performance levels, homeostatic responses to the activity, and potentially even the circadian timing of the individual [12-15,36-38]. As examples: 1) There is a diurnal rhythm to maximum achievable exercise performance levels with peaks typically observed in the afternoon and evening [36,37]; 2) Differential metabolic substrate utilization in response to exercise may occur depending on timing of exercise in relation to other exposures (such as feeding or fasting states) and/or concerning circadian/internal time [12,13,38]; 3) There is evidence in humans that exercise can phase shift circadian rhythm [14,15]. Conceivably, all three facets of timed exercise effects may be of benefit to the cancer patient. For instance, timed exercise may enhance circadian rhythm alignment in the cancer patient, which may lead to improved tolerability and efficacy of appropriately timed anticancer drugs (Figure 1). Wherein there is difficulty in performing exercise more generally, accounting for the time of day of the peak in performance may increase the exercise capability of the patient. Differential homeostatic responses to exercise depending on time of day and/or in relation to other exposures and homeostatic states (e.g., metabolic substrate utilization) may be useful in constructing strategies against e.g., cancer-induced cachexia [12,39].
OPPORTUNITIES FOR RESEARCH
Given the large and increasing burden of cancer, investigation of the different facets of timed exercise as adjunctive therapeutic strategies are warranted. To this end, we provide recommendations: Firstly, exercise interventions in cancer co-treatment should include using parameters of treatment efficacy as primary endpoints such as tumor response [Response Evaluation Criteria in Solid Tumors (RECIST)] in solid tumors, CT tumor density for tumors treated with molecular targeting agent, and immune-checkpoint blockade and atypical response patterns for cancer immunotherapy, etc [40]. Secondly, both observational and interventional studies should capture the circadian timing (or at least time of day) of exercise in addition to individual chronotype information (indicative of whether peaks/troughs in rhythms are likely to occur earlier or later in the day). Ideally, this will be done using wearable accelerometers. Indeed, wearables such as Actigraph (Pensacola, FL, USA) [41], GENEActiv (Cambridge, UK) [42], and Axivity (Newcastle, UK) [43] can be used to capture data related to intensity, frequency, duration, and timing of physical activity (Figure 2). It is expected that detailed drug treatment data including dose, frequency, time of administration, completion rate, dose reductions, side effects, specific treatment responses, and patient-reported outcomes should also be collected. Whether observational or interventional in design, such studies would add to the knowledge base on whether specifically timed exercise could be a viable and valuable adjunctive therapeutic strategy against cancer. Thirdly, it would be beneficial for studies investigating the circadian-associated effects of exercise on cancer treatment to collect biological samples. Biological samples will provide opportunities to study pathways associated with physiological responses of the human body to specifically timed exercise during cancer treatments.
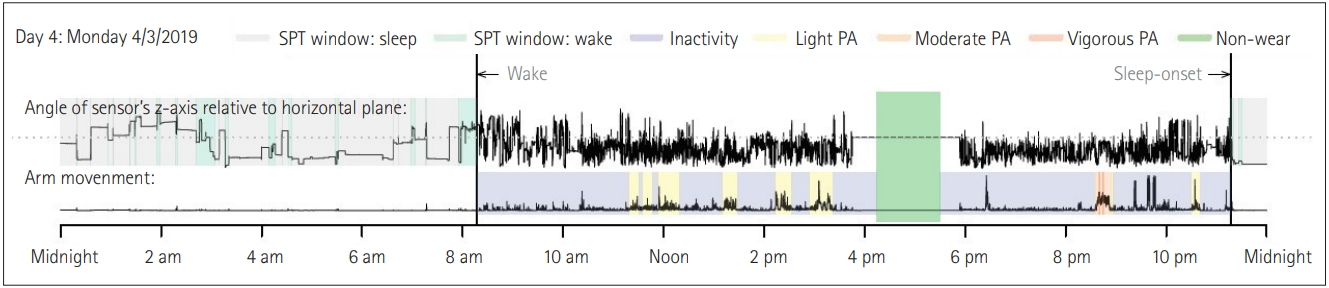
An example of the visual summary of accelerometer measured 24-hour temporal rest-activity patterns.SPT: sleep period time, PA: physical activity. Data processed by GGIR R-package [46], available online: https://cran.r-project.org/web/packages/GGIR/vignettes/GGIR.html.
Additionally, existing studies that have collected accelerometer data among cancer survivors or patients undergoing cancer treatment could potentially be revisited to generate parameters that facilitate exploratory analyses. Finally, beyond exercise but in a similar vein, studies involving the timing of food may be warranted given that food timing may also affect the human circadian system and circadian rhythm may also affect the homeostatic response to food (i.e., the fate nutrients) [44,45].
CONCLUSION
Research activities in exercise oncology recently began to investigate the effect of exercise on cancer treatment efficacy. Given that timing of exercise can have a diverse array of potential effects with relevance to cancer patients, studies that incorporate timing are warranted and may lead to innovative strategies in the fight against cancer.
Acknowledgements
None
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Lin Yang, Yikyung Park. Data curation: Lin Yang. Investigation: Lin Yang, Philip Lewis, Yikyung Park. Methodology: Lin Yang, Philip Lewis, Yikyung Park. Project administration: Lin Yang. Resources: Lin Yang. Supervision: Yikyung Park. Visualization: Lin Yang, Yikyung Park. Writing—original draft: Lin Yang. Writing—review & editing: Lin Yang, Philip Lewis, Yikyung Park.