Recent Update of Diagnostic Testing for Adult Sleep Disordered Breathing; Home Sleep Testing and Telemedicine
Article information
Abstract
Obstructive sleep apnea (OSA) is the most common sleep-related breathing disorder, characterized by repeated disruption and reduction of airflow during sleep due to obstruction of the airway. Polysomnography (PSG) is the standard method for OSA diagnosis. However, PSG has some limitations, including inaccessible, labor-intensive, expensive, and requiring facilities for a comprehensive evaluation. Recently, various tools have been developed to overcome the limitations of PSG. This system classifies as the “Home sleep testing (HST)” and “Telemedicine” for sleep-disordered breathing. The goal of this development should be accurate, reliable, convenient, reproducible, safe for data preservation, and patient self-mounting. Several devices have been released through clinical validation for decades. This review article introduces the latest sleep diagnostic test device updates and the clinical efficacy of HST and telemedicine.
INTRODUCTION
Obstructive sleep apnea (OSA) is a sleep-related breathing disorder characterized by repetitive collapses of the upper airway during sleep [1]. The prevalence in the United States is approximately 15–30% in males and 10–15% in females, when OSA is considered as an apnea-hypopnea index (AHI) greater than 5 events per hour of sleep [1,2]. OSA can be suspected if patients with risk factors, such as obesity and old age, have symptoms of excessive daytime sleepiness, snoring, and choking or gasping during sleep. For the diagnosis of OSA, objective testing must be performed in conjunction with a comprehensive evaluation and adequate follow-up; overnight polysomnography (PSG) is the standard method for diagnosis [3].
However, PSG has some disadvantages, such as being inaccessible, labor intensive, expensive, and requiring facilities and laboratories. Recently, to overcome the disadvantages of standard PSG and because of the high prevalence of OSA, portable monitoring (PM) and home sleep testing (HST) devices have been introduced in selected patients [4,5]. The ideal HST should be accurate, reliable, convenient, reproducible, and safe for data retention and patient self-mounting. Recently, in addition to applying polysomnographic technology to the HST, new and diverse technologies have been developed. The purpose of this review article is to introduce the clinical efficacy of HST and the recent update of sleep diagnostic test devices.
PORTABLE MONITORING AND HOME SLEEP TESTING
Due to a large number of undiagnosed subjects and the economic burden of sleep apnea and long wait times for laboratory PSG, there have been tremendous attempts to develop PM and HST. This interest in HST has led to the development of portable monitoring algorithms. In 1994, the American Academy of Sleep Medicine (AASM) published a classification scheme for the technology used to diagnose OSA [6]. Sleep test devices were classified into type I–IV, based on the presence of the laboratory and the inspector, and the types of measurable items [6] (Table 1).
Type I PSG refers to standard, in-laboratory, attended studies with a full array of recording channels, including electroencephalography, electro-oculography, chin electromyography, electrocardiography, measures of airflow, respiratory effort, and oxygen saturation [6,7].
The clinical practice guidelines for diagnostic testing for adult sleep apnea by the AASM for the first time formulated a strong recommendation that both PSG and HST are appropriate diagnostic testing options for uncomplicated adult patients who are at increased risk of moderate to severe sleep apnea [3].
Several PMs are notable for their ability to calculate total sleep time. The Apnea Risk Evaluation System monitor (ARES, SleepMed, Inc.) more closely approximates total sleep time. In two validation studies, a reasonable agreement was found between this device and the in-laboratory PSG [8,9]. The WatchPAT device (Itamar Medical, Ltd.) uses a proprietary algorithm that combines peripheral arterial tonometry, oximetry, heart rate monitoring, and actigraphy to provide an estimate of total sleep time and to calculate a respiratory disturbance index. A meta-analysis has shown a reasonable correlation between sleep indices calculated by in-laboratory PSG and those calculated by using peripheral arterial tonometry devices [10,11].
The following are indications for unattended PM and HST (Table 2).
Figure 1 and Table 3 show a schematic view of PM and HST devices mainly used in Korea, and summarize their features.
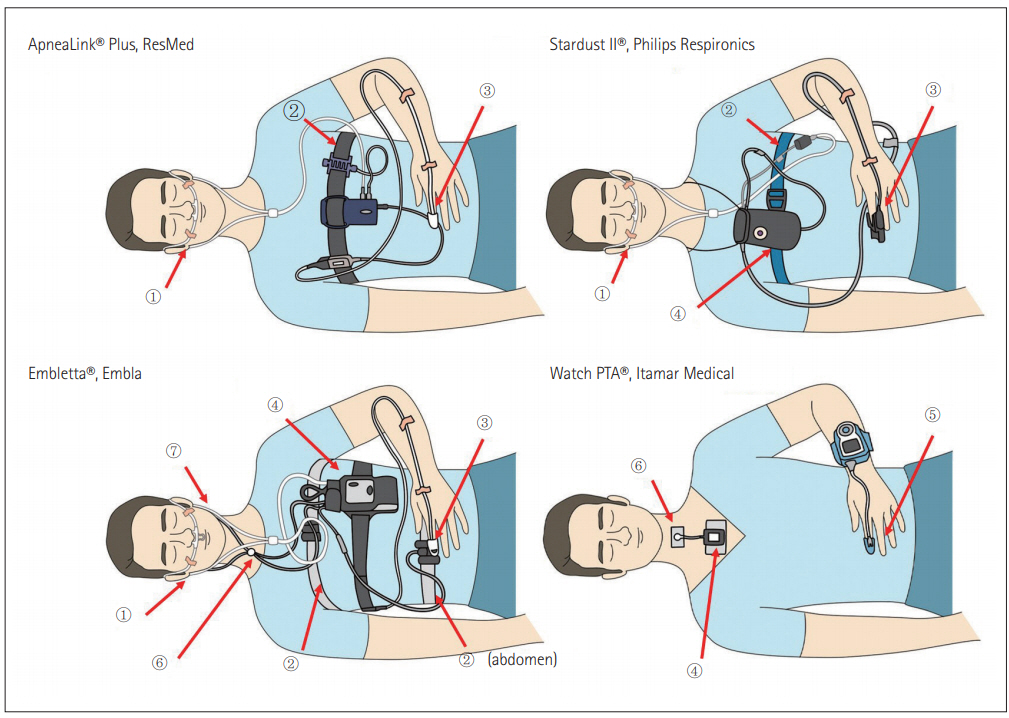
Portable monitoring (PM) and home sleep testing (HST) devices mainly used in Korea. <①: nasal pressure transducer, ②: respiratory effort belt, ③: pulse oximetry, ④: position sensor, ⑤: probe for PAT pulse oximetry, ⑤: Thermal sensor>, ⑥: Snore sensor.
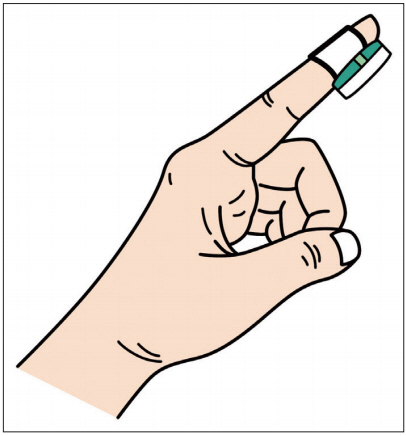
From another point of view, Collop et al. [4] reported that actuation with peripheral arterial tone (PAT) analysis and blood oxygen saturation (SpO2) combination could be a new diagnostic tool for OSA. Based on this theory, Massie et al. [12] proposed the NightOwlTM (Ectosense NV, Leuven, Belgium), which is a new system for the diagnosis of OSA from the edge of fingers. This design, which attaches the sensor to the fingertips with an adhesive patch, helps the patient apply for himself and start the analysis. Figure 2 illustrates the NightOwlTM. This system uses a cloudbased analysis platform. The sensor extracts the accelerometer and photoplethysmography data and draws PAT and blood oxygen saturation by software.
TELEMEDICINE
Telemedicine is a crucial factor in enhancing the treatment of patients with sleep-disordered breathing. In particular, telemedicine can help improve chronic respiratory disease. This is because regular inspections are an important requirement for testing whether the condition has exacerbated over the years. Current telemedicine systems enable self-care at home. Sleep doctors only need to explain how to handle the equipment. The device records the patient’s sleep time, breathing events, and positional effects on breathing during sleep. After the patient records the self-treatment, the sleep technician downloads the recorded data from the patient’s restored device. Sleep technicians create a scoring process using sleep data, a summary report with aggregated data is provided, and treatment decisions can be made [13].
This telemedicine has two different types: synchronous and asynchronous. The synchronous system is a real-time medical system. The system provides benefits such as expanding the treatment area of a sleeping doctor. It also improves continuous positive airway pressure training and troubleshooting through simple phone calls. The AASM has recognized the value of the expansion of telemedicine applications [14]. However, synchronous telemedicine has limitations that require an interview time with a doctor. According to the State of the American Telemedicine Association [15], the primary goal of telemedicine is to improve the accessibility, quality, and efficiency of treatment or cost-effectiveness. These synchronous systems can improve the accessibility and quality of treatment, but have little impact on treatment efficiency. However, asynchronous telemedicine can improve the limits of synchronous systems [14,16].
Asynchronous systems indicate that the meeting between the patient and the doctor does not occur in real time. This asynchronous system can be divided into several platforms: electronic messages, remote monitoring, automatic management, and self-management platforms. In detail, the electronic message indicates that an electronic mail and a text message are delivered to a patient in connection with medical information. The remote monitoring step first saves patient data from the medical test and reviews it later. The patient data stored in the medical device installed health information of the patient in a sleep state from a wearable sensor. The automatic management and self-management platform are fed back to the patient according to the treatment criteria of the smartphone application. This application can provide continuous care system. These components are key to help the asynchronous system’s ability to improve the therapeutic efficiency of sleep disorder breathing [16]. Telemedicine applications include recording sleep time and essential functions during telemedicine monitoring of sleep conditions and breathing, sleep apnea, and compliance with treatment regulations for patients with sleep apnea [17,18].
In recent technology, many modern diagnostic systems have been recommended for the treatment of sleep-disordered respiratory systems. For example, currently available devices provide wireless transmission of a recording module (e.g., oxygen saturation of a finger) to a body-worn on the chest using Bluetooth technology. This wireless connection between the body and the module is called the body area network. This technology improves patient comfort by providing body space from cable imprisonment. This means that the patient can self-apply using a recording device at home and avoid failures and connection problems. In addition, Bluetooth technology provides data transmission and continuous monitoring [18]. Currently, smartphones are considered one of the best options in diagnostic and therapeutic procedures for tracking or correcting sleep conditions because consumers typically use smartphones or tablet-based devices. In this case, the analysis of health information (exercise, breathing, heart rate, etc.) from the monitoring or historical data is completed by the application downloaded to the tablet or smartphone. The system allows consumers to save on system hardware costs (computing and storage). This means that the patient only needs to acquire a sensor. The application of these smartphone-based systems makes it extremely easy to use technology systems. In addition, these systems are available not only in medical centers but also in the health and lifestyle markets that provide general health assessments using online channels [18-20]. Additionally, physiological function was introduced to evaluate and classify telemedicine systems that can be used for sleep apnea. This assessment consists of detecting and recording sleep, cardiovascular, oxygen saturation, body position, breathing effort, and airflow. This scheme is called SCOPER [18,19]. This telemedicine-based system can improve the quality of diagnosis and treatment of sleep disorder breathing with patient convenience. In addition, modern wireless devices (used for Wi-Fi or Bluetooth systems) can be a key element in telemedicine research and clinical applications.
MONITORING SYSTEM BASED ON THE MATTRESS
The quality of life has continued to improve over the centuries. As development progresses, people pay more attention to personal health care. Sleep information management is gaining attention in academia and industry. However, traditional sleep monitoring systems are too complex and delicate for patients. To overcome this problem, several researchers have developed a noncontact mattress-based monitoring system. This mattress system can eliminate the external restrictions of the wearing device [21]. The first sensor systems were developed by Alihanka and Vaahtoranta [22]. They used pressure-sensitive foils underneath a bed to detect sleep, respiration, and heartbeat. This analysis system was confirmed by PSG. In 1989, this optimal system was introduced through signal filtering methods, signal processing, and signal acquisition [18]. In addition, other sensor systems using electromagnetic reflection have been developed to detect sleep conditions. This radio frequency can detect minute body movements triggered by heartbeats and breathing. Sleep doctors can optimize motion signal processing to distinguish sleep from wakefulness, sleep apnea, and normal breathing. Using this process, doctors can easily quantify sleep-wake scheduling disorders and start appropriate treatment early [23-25] (Figure 3).
Radar frequencies based non-contact sleep recording system. These systems detect body movements caused by respiration and heartbeat. Doppler technology with radar frequency improves detection performance and increases the measurement area. Reproduced from Penzel et al. New technology to assess sleep apnea: wearables, smartphones, and accessories. F1000Res 2018;7:413 [18], according to the Creative Commons Attribution Licence (CC-BY).
Recently, devices have been developed, including ceramic piezoelectric sensor data collection, data upload systems via Wi-Fi, processing data functions of host computers, and patient information displays. This update can detect physiological information of patient conditions during sleep every 30 s. This current update detects physiological information about the patient’s state of sleep every 30 s [21].
The sensor was also developed in a different way; for example, a pressure-based sensor mattress developed in Finland. This mattress is the first system that does not need to attach a sensor to the subject (Figure 4). This is called static charge sensitive bed. The sensor moves the ‘active layer’ of the mattress to detect body movements caused by breathing and heartbeat. The sensed intensity is converted in proportion to body movement and vibration [26]. However, this validation only used for an hour of sleep, but not a full sleep record [27,28]. In another study, this technique showed excellent performance in pediatric patients with obstructive apnea [29].
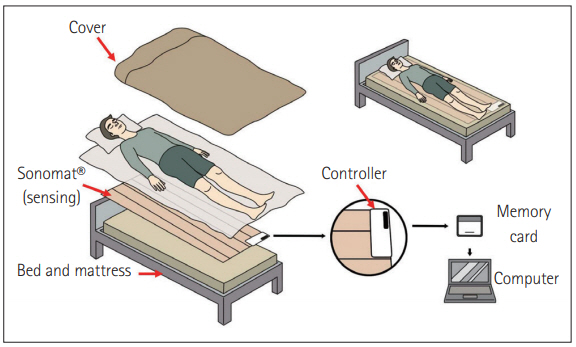
Schematic image of Sonomat®. Sonomat is placed directly on the mattress and covered with bedsheets. Patients just need sleep on the Sonomat without any equipment. The detection results are stored in the controller’s memory card. Results need to be transferred to a computer for analysis.
Australian researchers have developed Sonomat® (Sonomedical Pty. Ltd.), a technology-based acoustic data acquisition device (Figure 4). The system detects breathing movements and airflow in the form of breath sounds from four identical vibration/sound sensors. The acoustic data are scored in the same visualization as the PSG. The authors reported 90% accuracy, which is related to a commonly used diagnostic AHI blockade that has a 96.8% correlation with PSG. The drawback of this mattress system is only directly applied to a patient lying on the sensors. The authors mentioned that the patient was sometimes not lying on a sensor [30,31].
WEARABLE DEVICE
With the improvement of clouds and the Internet of Things, many researchers have attempted to develop various health care devices. In particular, wearable devices are attractive as a new generation technique, which helps to improve access to medical center, increase the treatment quality and cost-effect of diagnosis and treatment. Even through current development, most commercially available devices only apply to gather simple sleep information [32]. For example, HexoskinTM (Hexoskin) and Mag-IC system (Fondazione Don Carlo Gnocchi Onlus) is one of the smart shirt devices. It collects heart rate, total sleep time, and body movement. This type of shirt device can provide high mobility and comfort to patients [33,34]. RestEaZeTM (Tanzen Medical, Inc.) assumes the ankle band shape to overcome the limitation of wrist-worn devices such as diagnosis of restless leg syndrome and attention deficit hyper activity disorder, which are hard to detect from the wrist region. This device captures complex leg movement phenotypes with sleep fragmentation and short arousals [35]. OURATM (Oura Health Ltd.) is a smart ring that employs three types of sensors with infrared LEDs, a 3D accelerometer and gyroscope, and a temperature sensor to detect the sleep stages, heart rate, and body temperature. Based on this sensor, it can detect sleep time and sleep efficiency, including deep (PSG N3), light (PSG N1+N2), and rapid eye movement sleep [36]. In the future, this device will be developed for further application of OSA diagnosis.
CONCLUSIONS
Attended PSG is the gold standard test for diagnosing OSA. PM and HST with a technically adequate device may become an important modality for the diagnosis of OSA in uncomplicated and selected adult patients who indicate an increased risk of moderate to severe OSA. Telemedicine and wearable devices are feasible and challenging tools for the diagnosis of sleep-disordered breathing. Further studies are needed to clarify the indications and effectiveness verification of telemedicine and wearable devices.
Acknowledgements
We would like to thank Yoon Mi Shin for their kind help in the preparation of the figure work.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Seok Jin Hong. Writing—original draft: Sung Hun Kang, Seok Jin Hong. Writing—review & editing: Sung Hun Kang, Seok Jin Hong.