Relationship between Poor Sleep Quality and High White Blood Cell Count in Korean Adults
Article information
Abstract
Objective
This study aimed to investigate the relationship between sleep quality and white blood cell (WBC) count among Korean adults.
Methods
This cross-sectional study included 136 participants who attended the medical health check programs of two tertiary medical institutions from December 2019 to March 2021. Sleep quality was measured using the Korean version of the Pittsburgh Sleep Quality Index (PSQI-K). The participants were divided into the poor sleep quality group (n=94) and the good sleep quality group (n=42) according to the global PSQI-K score. A high WBC count was defined as that greater than the 75th percentile in the current sample. The odds ratios (ORs) and 95% confidence intervals (CIs) for high WBC count were calculated using multivariable logistic regression analyses after adjusting for confounding variables.
Results
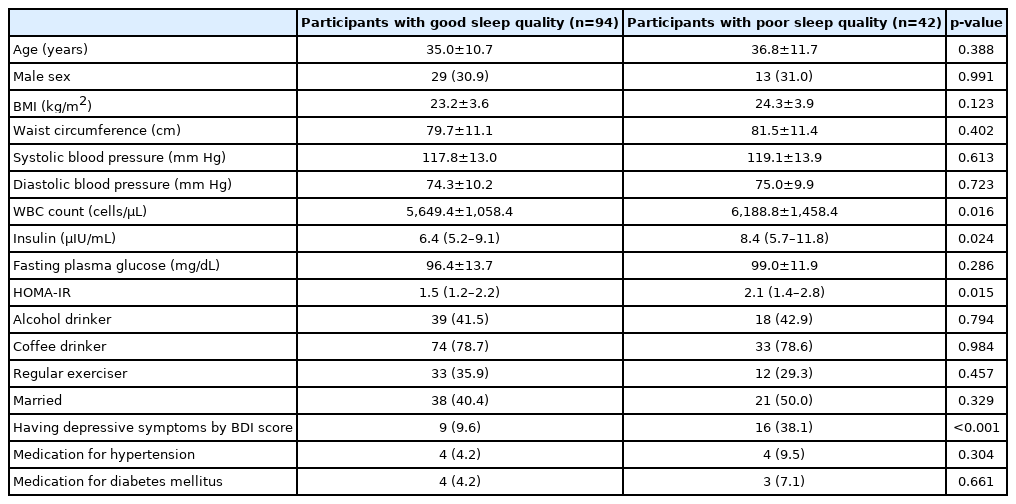
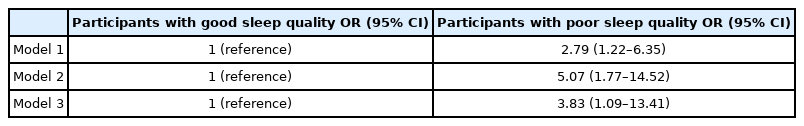
The mean WBC count was significantly lower in individuals with good sleep quality than in those with poor sleep quality. The prevalence of high WBC count was significantly higher in participants with poor sleep quality than in those with good sleep quality (p=0.018). Compared with the ORs (95% CIs) in individuals with good sleep quality, the ORs (95% CIs) for high WBC count in individuals with poor sleep quality were 3.83 (1.09–13.41) after adjusting for age, sex, alcohol consumption, coffee consumption, physical activity, marital status, hypertension, diabetes mellitus, depressive symptoms, body mass index, and homeostatic model assessment of insulin resistance.
Conclusion
Poor sleep quality was positively associated with an increased risk of high WBC count among Korean adults.
INTRODUCTION
Chronic low-grade inflammation has emerged as a central biological condition underlying the development of numerous medical disorders, including diabetes mellitus, cardiovascular diseases, neurodegenerative diseases, psychiatric disorders, and certain cancers [1,2]. Subclinical inflammation has also been associated with increased mortality [3]. The link between chronic low-grade inflammation and mortality-causing diseases indicates that the prevention of chronic low-grade inflammation is important from a public health perspective.
Sleep is a crucial factor that contributes to physical and mental health [4,5]. In addition, several studies have suggested that sleep quality can affect subclinical inflammation [6,7]. Although the exact mechanism between sleep and chronic low-grade inflammation is not fully understood, previous studies have shown that greater sleep disturbance was associated with higher levels of some inflammatory markers, such as interleukin-6 (IL-6) and C-reactive protein (CRP) [8-10]. In addition, several studies have revealed that experimental sleep deprivation caused an increase in the natural production of tumor necrosis factor-α (TNF-α) and IL-6 in monocytes [11-13].
However, IL-6, TNF-α, and CRP, which have been investigated in previous studies, are not routinely measured in clinical practice. In contrast, white blood cell (WBC) count, which is also known as a clinical marker of inflammation, is routinely tested in clinical practice. Few studies have examined the association between sleep quality and WBC count, and few studies have addressed the association between sleep quality and inflammatory markers in Koreans. Therefore, in this study, we aimed to investigate the relationship between sleep quality and WBC count in Korean adults.
METHODS
Study overview and study participants
This was a retrospective, cross-sectional study that included volunteers who were attending the medical health check programs at two institutions, the Severance Health Checkup and the Department of Family Medicine of the Gangnam Severance Hospital, Seoul, Korea, between December 2019 and March 2021. A total of 161 adults over 19 years of age were surveyed. We excluded individuals who had missing data or did not complete the questionnaire. Since smoking could result in a raised WBC count, we also excluded current smokers. Of the remaining participants, those with a WBC count <4,000 cells/μL or >10,000 cells/μL were excluded to rule out the possibility of bone marrow suppressive illnesses, infections, or inflammatory disorder. After these exclusions, 136 participants (42 men and 94 women) were included in the final analysis. Written informed consent was obtained from each participant. This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and was approved by the Institutional Review Board of Yonsei University Gangnam Severance Hospital (Institutional Review Board number: 3-2019-0226).
Covariates
Each participant completed a questionnaire that included questions regarding past medical history, marital status, smoking status, alcohol consumption, and coffee consumption. Alcohol drinker was defined as alcohol consumption on one or more days per week, and that of coffee was defined as consuming one or more cups a day. Physical activity was assessed using the Korean version of the International Physical Activity Questionnaire short form [14], and a regular exerciser was defined as a person who performed ≥20 minutes of vigorous physical activity ≥3 days a week or ≥30 minutes of moderate physical activity ≥5 days a week. Depressive symptoms were evaluated using the Beck Depression Inventory (BDI) questionnaire [15,16], and a person having depressive symptoms was defined as a person with a BDI score ≥10 [17,18].
Anthropometric measurements were obtained by two trained medical staff members in accordance with the standardized procedures in each of the two hospitals. Body weight and height were measured with participants wearing light indoor clothing and no shoes. Body mass index (BMI) was calculated as body weight (kg) divided by height squared (m2). Systolic and diastolic blood pressures were obtained from patients in the sitting position after a 10-min resting period. Blood samples were collected from patients following at least 8 hours of fasting. WBC count were measured using the XN9000 automated hematology analyzer (Sysmex, Kobe, Japan) and the ADVIA 2120i hematology analyzer (Siemens, Munich, Germany). Fasting plasma glucose was measured using the AU5800 autoanalyzer (Beckman Coulter, Brea, CA, USA). Serum insulin levels were measured using electrochemiluminescence immunoassay with Cobas E601 analyzers (Hoffman-La Roche, Basel, Switzerland).
The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated using the following formula: fasting plasma glucose (mg/dL) × fasting insulin (μIU/mL) / 405 [19].
Measurement of sleep quality
Sleep quality was measured using the Korean version of the Pittsburgh Sleep Quality Index (PSQI-K). This index is a self-administered questionnaire that includes 19 questions for assessing sleep quality over the previous month [20,21]. It is composed of seven subcategories: subject sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, use of sleeping medication, and daytime dysfunction. Each item in the PSQI-K is scored from 0 to 3, where 0 means the positive extreme and 3 means the negative extreme on the Likert scale; the global PSQI-K score is the sum of these items, with scores ranging from 0 to 21. Good sleep quality was defined as a global PSQI-K score of ≤5, and poor sleep quality was defined as a global PSQI-K score >5.
Statistical analysis
Normal distribution was evaluated by determination of skewness using the Kolmogorov–Smirnov test. Serum insulin and HOMA-IR values showed a skewed distribution. The characteristics of the study participants according to sleep quality were expressed as mean±standard deviation, median (interquartile range), or percentages and compared using independent t-test or Wilcoxon rank-sum test for the continuous variables and chi-squared test for the categorical variables. To evaluate the association between sleep quality and WBC count, we used multiple linear regression analyses. The odds ratios (ORs) and 95% confidence intervals (CIs) for high WBC count (>75th percentile in the current sample) were calculated using multivariable logistic regression analysis after adjusting for the confounding variables. All analyses were conducted using the SPSS statistical software (version 25.0; IBM Corp., Armonk, NY, USA). All statistical tests were two-sided, and statistical significance was determined at a p-value <0.05.
RESULTS
Table 1 shows the characteristics of the study participants according to their sleep quality. The number of participants with good sleep quality and poor sleep quality was 94 and 42, respectively. The mean or median values of WBC count, insulin, and HOMA-IR were significantly lower in individuals with good sleep quality than in those with poor sleep quality.
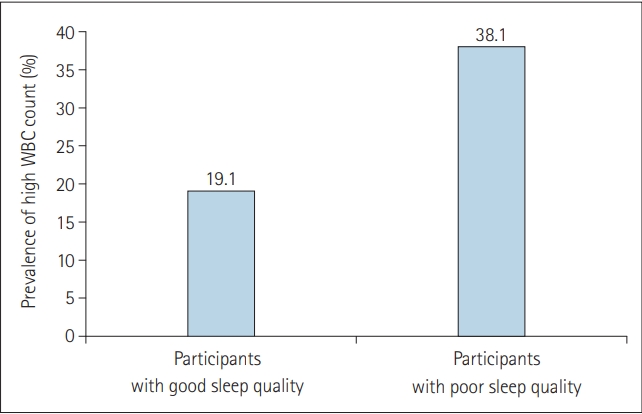
Figure 1 presents the prevalence of high WBC counts according to sleep quality. The prevalence of high WBC count was significantly higher in participants with poor sleep quality than in those with good sleep quality (p=0.018).
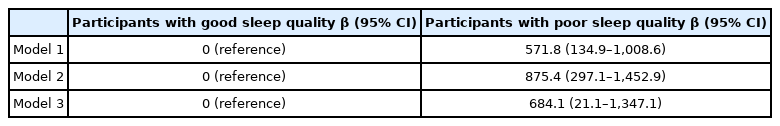
Table 2 presents the results of linear regression analyses that were used to investigate the association between sleep quality and WBC count. Sleep quality showed statistically significant associations with WBC count in multiple linear regression analyses. The associations between sleep quality and WBC count remained significant in multiple regression analyses that were adjusted for confounding factors. Compared with the participants with good sleep quality, the participants with poor sleep quality were associated with adjusted β-coefficient (95% CIs) for WBC count of 684.1 (21.1–1,347.1).
Table 3 shows the ORs (95% CIs) of high WBC count according to sleep quality, determined using multiple logistic regression analyses. In Model 1, the ORs (95% CIs) were calculated after adjusting for age and sex. In Model 2, we adjusted for additional potential confounding variables, such as alcohol consumption, coffee consumption, physical activity, marital status, hypertension, and diabetes mellitus. In Model 3, we investigated the association between sleep quality and high WBC count, independent of depressive symptoms, obesity effects, and insulin resistance, afteradjusting for additional confounding variables, such as having depressive symptoms by BDI score, BMI, and HOMA-IR. Compared with the ORs for individuals with good sleep quality, the ORs (95% CIs) for high WBC count in individuals with poor sleep quality was 2.79 (1.22–6.35) in Model 1, 5.07 (1.77–14.52) in Model 2, and 3.78 (1.07–13.33) in Model 3.
DISCUSSION
In the current study, we found that poor sleep quality was significantly associated with a high WBC count in Korean adults after adjusting for potential confounding variables. This association was maintained regardless of the effects of depressive symptoms, obesity, and insulin resistance, even after controlling for having depressive symptoms by BDI score, BMI, and HOMA-IR. While there were some inconsistencies, most of the previous studies have shown a relationship between poor sleep quality and inflammatory markers. A meta-analysis of 72 studies reported that greater sleep disturbance was related to higher levels of circulating inflammatory markers, such as IL-6 and CRP [10]. Our findings are consistent with the results of these previous studies, showing an association between poor sleep quality and inflammatory markers. Moreover, our results suggest that poor sleep quality is associated with a high WBC count, a test which is affordable, easy to interpret, and ordered routinely in clinical practice. A previous study revealed a relationship between sleep quality and CRP in Korean adults [8]. However, CRP, the inflammatory marker used in that study, is not routinely measured in clinical practice. Furthermore, the questionnaire used in that study for assessing sleep quality was composed of only two questions, while the present study used the PSQI-K, which is widely used for its reliability and validity for assessing sleep quality [21]. Thus, our results expand on earlier findings regarding the association between sleep quality and inflammatory markers.
Several possible mechanisms could underlie the significant association between sleep quality and high WBC count, one of the inflammatory markers. The sympathetic nervous system (SNS) and hypothalamic-pituitary-adrenal (HPA) are the major effector systems related to sleep and inflammatory markers [22]. SNS activity decreases markedly during sleep [23], accompanied by vagal outflow or a shift from sympathetic to parasympathetic with sleep onset [24]. The nocturnal fall in SNS activity is hindered when sleep disturbance occurs, resulting in an overall increase in SNS activity during the night [18], and these alterations are carried over into the daytime [25,26]. Individuals with insomnia also experience increased sympathetic outflow accompanied with increased levels of circulating adrenaline and noradrenaline, which is also related to increased levels of inflammatory markers [27]. In addition to SNS, HPA has an effect on the systems that link sleep and inflammatory markers. Sleep disturbances lead to activation of the HPA axis [28,29]. Consequently, repeated HPA axis activation can generate glucocorticoid-resistant immune cells [30-32]. Thus, both inflammation and HPA axis activation occur in people with sleep disturbances [33].
Another important factor to consider in the context of inflammation is insulin resistance. Insulin resistance is increasingly being perceived as a chronic low-grade inflammatory status, and poor sleep quality is associated with insulin resistance [34,35]. Indeed, in this study, median value of HOMA-IR (a measure for quantifying insulin resistance) was significantly higher in individuals with poor sleep quality than in those with good sleep quality. Based on this evidence, the interrelationship between sleep quality and inflammation may be explained by insulin resistance.
This study had several limitations. First, this study used a crosssectional design. Therefore, caution should be exercised in causal and temporal interpretations, and additional longitudinal studies are needed. Second, sleep quality was evaluated using a subjective tool instead of an objective tool, such as polysomnography. However, the PSQI-K is a reliable and valid questionnaire for assessing sleep quality and can be useful for differentiating between individuals with good sleep quality and poor sleep quality [21]. Third, only one WBC count measurement was included in the analysis; thus, it was not possible to determine whether an acute and brief episode of infection affected the findings reported herein. To minimize this limitation, subjects with a WBC count <4,000 cells/μL or >10,000 cells/μL were excluded to minimize this limitation. Fourth, although WBC count can be affected by stress, we did not measure the stress levels of participants. Fifth, when we considered alcohol consumption, we considered only the frequency of alcohol intake. Further studies are needed to consider the amount of alcohol consumed along with the frequency of intake. Sixth, since participants were enrolled in the two hospitals, WBC counts were measured with two hematology analyzers. However, these analyzers showed a good concordance for the basic blood count parameters [36]. Seventh, this study used only WBC count as an inflammatory marker. Further studies are warranted to investigated the association between sleep quality and various inflammatory marker, including CRP. Lastly, the small number of participants may not have been representative of the general population in Korea. Despite these limitations, we believe that this is the first study to demonstrate an association between poor sleep quality and high WBC count. Furthermore, a wide range of confounding factors closely related to poor sleep quality and subclinical inflammation, including age, sex, alcohol consumption, coffee consumption, physical activity, marital status, hypertension, diabetes mellitus, depressive symptoms, BMI, and HOMA-IR, were adjusted for in multiple logistic regression analyses.
In conclusion, poor sleep quality was positively associated with an increased risk of high WBC count in Korean adults. The present study contributes to our understanding of the association between sleep quality and inflammatory markers. The findings of this study may have clinical implications regarding the public health strategies that prevent chronic low-grade inflammation.
Acknowledgements
This work was supported by the Technology Innovation Program (20002781, Platform for Prediction and Management of Health Risk Based on Personal Big Data and Lifelogging) funded by the Ministry of Trade, Industry & Energy (MOTIE, South Korea).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: all authors. Data curation: all authors. Formal analysis: all authors. Funding acquisition: Ji-Won Lee. Investigation: all authors. Methodology: all authors. Project administration: Ji-Won Lee. Resources: all authors. Software: all authors. Supervision: Ji-Won Lee. Validation: all authors. Visualization: all authors. Writing—original draft: all authors. Writing—review & editing: all authors.