No Association Between the 5-HTTLPR Polymorphism and Diurnal Preference in Koreans
Article information
Abstract
Objective
Recent studies have extended the study of diurnal preferences to the genetic level. Most studies have focused on clock genes, but some studies have searched for the possibilities of other genes associated with the circadian rhythm. Considering that the circadian rhythm is associated with the serotonergic system, investigating the association between major genes of the serotonin system and the diurnal preference phenotype is essential. In this study, we evaluated whether the 5-HTTLPR polymorphism is associated with diurnal preference in a Korean population.
Methods
In total, 509 healthy subjects were genotyped for the 5-HTTLPR polymorphism. The Korean version of the Composite Scale of Morningness (CSM) was used to measure the phenotype patterns of diurnal preference. In addition, scores of three subscales—morningness, activity planning, and morning alertness—were extracted from the CSM.
Results
No significant associations were observed between CSM scores and the 5-HTTLPR genotype or allele carrier status.
Conclusion
The results of this study suggests that 5-HTTLPR has no effect on diurnal preference in a healthy Korean population. Further studies with a large number of subjects from multiple ethnicities are necessary to fully evaluate the association between 5-HTTLPR and diurnal preference.
INTRODUCTION
Diurnal preference is a circadian typology consisting of three chronotypes—morning, evening, and neither types. Morningtype individuals sleep and wake up early and achieve most of their peak mental and physical performance in the early part of the day. In contrast, evening-type individuals sleep and wake up late, showing their best performance mostly during the evening hours [1]. According to many studies, compared to morning-type individuals, evening type individuals have more negative health consequences, such as mood disorders, anxiety disorders, substance use disorders, autism, personality disorders, insomnia, sleep apnea, hypertension, diabetes, and infertility [2]. Diurnal preference has interpersonal variations due to differences in age, sex, and genetic and environmental factors that could influence circadian rhythms [3,4].
The circadian rhythm is a roughly 24-hour cycle related to the sleep/wake cycle, locomotor activity rhythm, and the secretion rhythm of various hormones. It is endogenously generated by a defined neural network located in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus and various “clock genes,” which drive the neural circadian rhythms through daily autoregulatory transcription/translation feedback oscillations [5,6].
In addition to clock genes, the circadian rhythm is associated with the serotonergic system in the brain [7]. The brain’s serotonergic system is located in the midline raphe nucleus of the midbrain, pons, and medulla and contains an extensive network of efferents to the cortex, thalamus, hypothalamus, and limbic system.
The circadian and serotonergic systems are extensively intertwined in both neuroanatomical and physiological aspects. The SCN receives direct serotonergic innervation from the raphe nucleus and indirect dorsal raphe-driven neuropeptide Y input from the intergeniculate leaflet. The dorsal raphe nucleus receives input back from the SCN [8]. Serotonin, a neurotransmitter, not only modulates the response of the circadian system to light by inhibiting retinal inputs to the SCN neurons but also affects the circadian phase via behavioral arousal and feedback from locomotor activity [9,10]. The brain’s serotonergic system operates in a circadian manner, and many molecules of the serotonin signaling network, such as SERT, 5-HT1B, 5-HT2C, and 5-HT7 receptors, are expressed in the SCN [7]. Tryptophan hydroxylase, the rate-limiting enzyme for serotonin synthesis, and the serotonin concentration in the raphe nucleus is regulated by the circadian rhythm [11].
Recent studies have extended the study of diurnal preferences to the genetic level. Circadian genes, such as CLOCK, PER2, PER3, CRY1, ARNTL, and RORa, have been investigated for their association with diurnal preference, but many studies did not find any statistical significance or failed to replicate previous positive finding [12-16]. Yeom et al. [17] suggested that HTR2A, a gene encoding one of the serotonin receptors, is associated with diurnal preference. Considering the close relationship between the serotonergic and circadian system, this finding seems to be reasonable and leads to the possibility that some other serotonergic genes are associated with diurnal preference.
One candidate for such an investigation is 5-HTTLPR. 5-HTTLPR is a promoter region of SLC6A4, a gene encoding the serotonin transporter. The polymorphism of 5-HTTLPR involves two common allelic variations—the short (S) allele and the long (L) allele. Compared to the L allele, the S allele is associated with a lower expression of the serotonin transporter (5-HTT), which reuptakes serotonin, resulting in an increase in synaptic serotonin concentration [18]. Studies suggest that the S allele is associated with individuals’ psychological and physiological responses to life stressors and is clinically associated with various neuropsychiatric disorders, including mood disorders, seasonal affective disorders, anxiety personality traits, substance use disorders, attention deficit hyperactivity disorder, and autism [19-24].
Given the effect of the serotonin system on the circadian rhythm, further investigation of the association between major genes of the serotonin system and the diurnal preference phenotype is essential. Furthermore, marked similarity between the range of disorders associated with the evening type of the diurnal preference and the 5-HTTLPR S allele makes 5-HTTLPR a good candidate gene for research. In this study, we examined the association between diurnal preference and the 5-HTTLPR polymorphism in healthy Korean adults. We assessed the effects of genotype and allele carrier status on the mean total score of the Korean version of the Composite Scale of Morningness (CSM) and the mean scores of its three subscales (morningness, morning alertness, and activity planning). We hypothesized that the 5-HTTLPR is associated with the evening type of diurnal preference, indicated by low mean total and subscale scores of the CSM.
METHODS
Subjects
A sample of 510 healthy Korean adults was recruited through an online advertisement, and one participant was excluded due to missing information. Participants were unrelated Korean adults who resided in Seoul (37°41´N, 127°02´E) aged 18–35 years (mean ±standard deviation [SD], 23.4±2.8 years). There were 302 (59.3%) men and 207 (40.7%) women. Participants were confirmed to be free of lifetime or current psychiatric disorders by an experienced psychiatrist through a mini-international neuropsychiatric interview. The study participants did not have any major medical problems. Participants with a family history of substance abuse or major psychiatric disorders (e.g., schizophrenia or major mood disorders) were excluded. All participants provided informed consent prior to enrollment in the study. The study protocol was approved by the Ethics Committee of Korea University (IEC No. 1067), and the study was conducted in accordance with the Declaration of Helsinki. Other findings related to these participants have been reported previously [12,16,17,25-27].
Diurnal preference assessment
Diurnal preference was measured using the CSM [28]. The CSM is a 13-item scale that is considered as accurate as the Horne and Ostberg’s Morningness–Eveningness Questionnaire, but it is shorter in length [28,29]. The CSM is an added-score scale with items, with scores ranging from 1 to 4 or 5. The total score of the CSM ranges from 13 (extreme evening type) to 55 (extreme morning type), with higher scores indicating a greater degree of morning preference. According to previous studies, CSM items can be further classified into three subscales—morningness (items 1, 3, 6, 8, 10, and 11), activity planning (items 2, 7, 9, and 13), and morning alertness (items 4, 5, and 12) [30]. All participants were asked to complete the Korean version of the CSM verified by Yoon et al. [31].
Genotyping
Genomic DNA was extracted from leukocytes using the QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). For the polymerase chain reaction (PCR) analysis of 5-HTTLPR, the forward primer 5'-GGC GTT GCC GCT CTG AAT TGC-3' and reverse primer 5'-GAG GGA CTG AGC TGG ACA ACC CAC-3' were used. PCR amplification was performed using a 25 μL solution containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM of each dNTP, 0.25 μM of each primer, 1 U of Taq DNA polymerase, and 40 ng genomic DNA. The conditions used for PCR amplification included initial denaturation at 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, 60°C–61°C for 30 s, 72°C for 40 s, and final elongation at 72°C for 7 min. PCR products were separated on 3% agarose gels supplemented with ethidium bromide to allow identification of the long (528 bp) and short (484 bp) variants.
Statistical analysis
The chi-square (χ2) test was used to test the goodness-of-fit of the Hardy–Weinberg equilibrium. Analysis of covariance (ANCOVA) with sex and age as covariates was performed to examine the effects of genotypes on the mean total CSM score and mean scores of the three CSM subscales (morningness, activity planning, and morning alertness). To examine the effects of S allele carrier status (allele carriers 1 in Table 1) and L allele carrier status (allele carriers 2 in Table 1) on the mean total CSM and its subscale scores, ranked ANCOVA and ANCOVA with sex and age as covariates were performed, respectively. A two-tailed test with alpha=0.05 was chosen for the analysis. All statistical analyses were performed using SPSS version 22.0, for Windows (IBM Corp., Armonk, NY, USA).
RESULTS
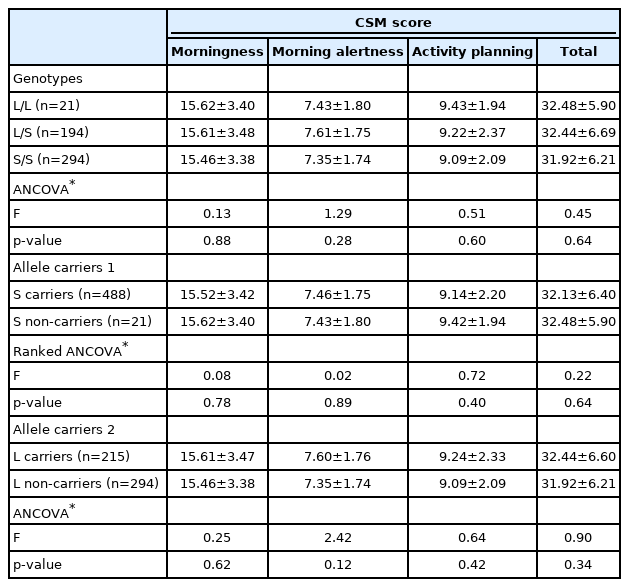
The total CSM mean score was 32.14±6.38, with a range of 14 to 51. Table 1 shows the mean of the total CSM score and the three subscale scores of the groups classified based on the 5-HTTLPR polymorphisms, either divided by genotype or allele carrier status. The genotypic distributions of the 5-HTTLPR polymorphisms in the enrolled subjects were in Hardy–Weinberg equilibrium, and no significant differences were found between the observed and expected values (χ2=2.50, p=0.29). In addition to genotype comparisons, the study subjects were compared according to their allele carrier status. The frequency of the S allele at 5-HTTLPR in our sample population was 76.8%.
No significant differences were observed in the mean total CSM scores and mean scores of the three subscales among the genotypes of the 5-HTTLPR polymorphisms (F=0.45, p=0.64 for total CSM; F=0.13, p=0.88 for morningness; F=1.29, p=0.28 for morning alertness; F=0.51, p=0.60 for activity planning). There were no significant differences between S carriers and non-carriers (F=0.22, p=0.64 for total CSM; F=0.08, p=0.78 for morningness; F=0.02, p=0.89 for morning alertness; and F=0.72, p=0.40 for activity planning) and between L carriers and non-carriers (F=0.90, p=0.34 for total CSM; F=0.25, p=0.62 for morningness; F=2.42, p=0.12 for morning alertness; and F=0.64, p=0.42 for activity planning).
DISCUSSION
The aim of the present study was to investigate the association between the 5-HTTLPR polymorphism and diurnal preference; however, no statistically significant results were found.
To the best of our knowledge, this is the first study to investigate the association between 5-HTTLPR and diurnal preference in an Asian population. Previous studies on the relationship between 5-HTTLPR and diurnal preference have reported inconsistent findings. Ojeda et al. [32] found a significant association in a sample of 191 young South American adults, while Barclay et al. [33] found no such association in 947 young British adults. Both studies used linear regression analysis corrected for sex and age. In Barclay’s study, the interaction of negative life events was additionally considered, but it did not change the result. Such inconsistencies in previous findings could be explained by two reasons. First, as Barclay claimed, the sample sizes of the previous studies may have been limited, and a larger sample might be needed to confirm a solid conclusion [33]. Second, it may be due to differences in ethnicity, culture, and social environment of the sample population, which may influence the 5-HTTLPR effect. For instance, ethnicity was found to moderate the association between 5-HTTLPR and national suicide rates; the S allele was a protective factor in Caucasians and a risk factor in non-Caucasian populations [34]. Further studies with a large number of subjects from multiple ethnicities and cultural factors are necessary to fully evaluate the association between 5-HTTLPR and diurnal preference.
The current study has several limitations. First, population stratification bias cannot be excluded. However, since the Korean population shows a relatively high degree of genetic homogeneity and the enrolled subjects consisted of healthy young Korean adults, the possibility of population stratification bias in this study seems unlikely [35]. Second, although our sample size was sufficient for statistical significance, it may not be sufficient to test a single-nucleotide polymorphism effect underlying the complexity of diurnal preference. Third, our study evaluated the two variants of the 5-HTTLPR polymorphism, S allele and L allele, which may obscure the more intrinsic interaction and effect of other 5-HTTLPR polymorphisms. The assumed functionally bi-allelic 5-HTTLPR polymorphism may not accurately represent the true association between 5-HTTLPR and diurnal preference [36]. Fourth, environmental effects or stress events that might influence the subject’s diurnal preference or effect of 5-HTTLPR were not considered in this study. Although diurnal preference is largely genetic, socialenvironmental factors also affect diurnal preference [37]. And though controversial, several meta-analyses found a significant gene-environment interaction between stress and 5-HTTLPR polymorphism [38,39]. Although most of our participants were college students, no information about involving shift-working jobs or assessment of life stress events was not included.
In conclusion, this study did not find a statistically significant association between the 5-HTTLPR polymorphism and diurnal preference in the Korean population. Due to limited research, our understanding of the genetic effects associated with diurnal preference is inadequate. Future studies may find additional genes associated with diurnal preference and improve our understanding of the genetic effects on the phenotypic expression of circadian rhythmicity. As many psychiatric disorders are associated with circadian rhythms, further research may lead to valuable treatment strategies.
Acknowledgements
This study was supported by the Korea Health 21 R&D Project funded by the National Research Foundation of Korea (2017M3A9F1031220 and 2019R1A2C2084158).
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Heon-Jeong Lee. Data curation: Ju Yeon Seo, Ji Won Yeom, Sehyun Jeon. Formal analysis: Sae Han Kim, HeonJeong Lee. Funding acquisition: Heon-Jeong Lee. Investigation: Sae Han Kim, Seunghwa Jeong. Methodology: Sae Han Kim, Seunghwa Jeong, Ji Won Yeom, Sehyun Jeon. Project administration: Ju Yeon Seo, Heon-Jeong Lee. Resources: Heon-Jeong Lee. Software: Sae Han Kim, Seunghwa Jeong, Ji Won Yeom, Sehyun Jeon. Supervision: Heon-Jeong Lee. Validation: Heon-Jeong Lee. Visualization: Sae Han Kim. Writing—original draft: Sae Han Kim, Writing—review & editing: Heon-Jeong Lee.