Clinical Characteristics of HIV-Infected Patients With Sleep Disturbance
Article information
Abstract
Objective
This study aims to examine clinical characteristics and to identify possible risk factors of sleep disturbances in people living with HIV (PLWH).
Methods
All research data of patients who were first diagnosed with HIV/AIDS from January 1, 2012 to December 31, 2021 and complained of sleep disturbance at least once were retrospectively reviewed by the Severance Clinical Research Analysis Portal (SCRAP) service of Severance hospital. The presence of sleep disturbance was evaluated based on whether insomnia disorder diagnosis code was included or whether insomnia medication was prescribed. The patients were divided into either the group with sleep disturbance within 3 months (SDW3) and the group with after 3 months (SDA3). All data were reported using descriptive statistics.
Results
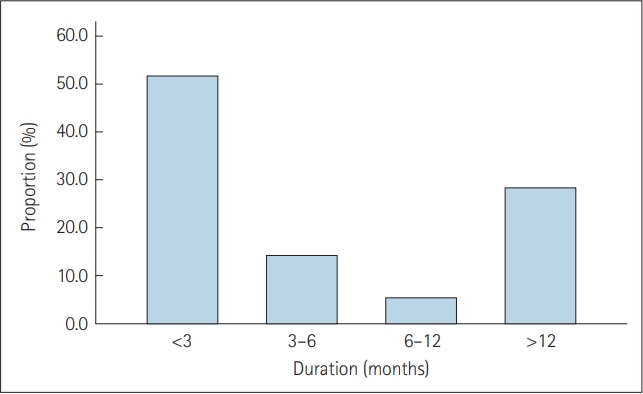
Of the 674 patients diagnosed with HIV during the period, 56 patients experienced sleep disturbances at least once and approximately 50% of patients have experienced sleep disturbance in the first 3 months after HIV diagnosis. CD4+ cell count at the time of first onset of sleep disturbance was significantly lower (p=0.03) and HIV viral load at the time of first onset of sleep disturbance was significantly higher (p<0.001) in SDW3 group. SDW3 patients showed higher rates of opportunistic infections compared to SDA3 patients.
Conclusion
The current study suggests that further investigation of the underlying pathophysiology of sleep disturbance and association with immunological changes for early diagnosis and treatment of sleep disturbance in PLWH.
INTRODUCTION
Sleep disturbances appear to be more common among people infected with human immunodeficiency virus (HIV) than the general population [1]. The prevalence rate of self-reported sleep disturbance including insomnia, obstructive sleep apnea, and poor sleep quality in people living with HIV (PLWH), was estimated to be 58% in previous meta-analysis [2]. Sleep disturbances have been reported at all stages from the beginning of HIV infection to progression to acquired immunodeficiency syndrome (AIDS) [3]. Given that the focus on HIV has shifted from patient survival to controlling co-morbidities and improving quality of life, due to the development of antiretroviral therapy (ART), sleep disturbances in PLWH have become clinically important due to potential impact on quality of life, comorbid psychiatric symptoms including depression [4] or anxiety [1] and adherence to ART [5]. Recent studies have largely focused on the impact of certain antiretroviral drugs such as efavirenz [6] and dolutegravir [7] on sleep quality. In addition, considering the regulatory role of sleep in the immune function, poor sleep has been associated with disease progression in HIV specifically [8].
Although the pathophysiology of sleep disturbances among PLWH remains not well understood, the direct effects of HIV in the central nervous system (CNS) which is related to interference of circadian system [9], side effect of antiretroviral medications [10,11], CNS opportunistic infections [12], immunological response to HIV infection [13], psychological factors including perceived stress [14], depression [15], and anxiety [16] are thought to impact the pathophysiological processes. Regardless of its etiology, however, sleep disturbances in PLWH are clinically important for several reasons that sleep disturbances not only impair daytime functioning, but also are associated with metabolic dysregulation and cardiovascular disease if they persist chronically [13].
Despite contribution to quality of life and disease progression, sleep disturbances remained underdiagnosed and undertreated in PLWH [17]. Further, studies on sleep disturbance among PLWH remain scarce and to the best of our knowledge, no study has been carried out in South Korea for the high prevalence and clinical significance. Thus, this study aims to examine clinical characteristics and to identify possible risk factors of sleep disturbances in PLWH.
METHOD
Study design and participants
We conducted a retrospective chart review study of patients with HIV/AIDS and sleep disturbance at Yonsei University College of Medicine Severance Hospital. This study reviewed all charts of patients who were first diagnosed with HIV/AIDS from January 1, 2012 to December 31, 2021 and complained of sleep disturbance at least once. The presence of sleep disturbance was evaluated based on whether insomnia disorder diagnosis code was included or whether insomnia medication was prescribed. Patients were excluded if patients were diagnosed with HIV/AIDS before 2012 by institutions other than Yonsei University College of Medicine Severance Hospital or if patients were prescribed medications for sleep disturbance or had insomnia disorder diagnosis codes before HIV diagnosis. To investigate the difference in demographic and clinical characteristics of sleep disturbance according to the duration from HIV diagnosis to onset of sleep disturbance, the patients were classified into either the group with sleep disturbance within 3 months of HIV diagnosis (SDW3) and the group with sleep disturbance after 3 months of HIV diagnosis (SDA3). The study was approved by the Institutional Review Boards at Severance Hospital of the Yonsei University Health System, Seoul, Korea (IRB approval no. 4-2022-0851). Given the retrospective nature of the study, informed consent was waived.
Data collection
All data were retrieved from the Severance Clinical Research Analysis Portal (SCRAP) service of Severance Hospital. Patients who had sleep disturbances were selected if hypnotic drugs were prescribed at least once or if one of these insomnia-related International Classification of Diseases, tenth revision (ICD-10) codes were applied as either principal or an additional diagnosis at least once after HIV diagnosis [18]; nonorganic insomnia (F51.0), nonorganic hypersomnia (F51.1), other nonorganic sleep disorders (F51.8), nonorganic sleep disorder, unspecified (F51.9), disorders of initiating and maintaining sleep (G47.0), disorders of excessive somnolence (G47.1), obstructive sleep apnea (G47.30), central sleep apnea (G47.31), mixed sleep apnea (G47.32), other and unspecified sleep apnea (G47.38). The hypnotic drugs included zolpidem, eszopiclone, and triazolam. These medications were selected because they are all commonly used for insomnia, but rarely for antidepressants or anxiolytics. Benzodiazepines other than triazolam were excluded because those are widely used not only for insomnia but also for anxiety. Demographic variables including age at HIV diagnosis, sex, level of education, sexual orientation, and socioeconomic status were collected. Clinical variables collected include date of HIV diagnosis, CD4 count and viral load at HIV diagnosis and at the time of the first onset of sleep disturbance, ART when sleep disturbance occurs for the first time, psychiatric consultation status, past psychiatric history, presence of psychiatric consultation, and medical diseases at HIV diagnosis.
Data analysis
All data were reported using descriptive statistics. Categorical variables were summarized by percentages and continuous variables were reported by means and standard deviation (SD). The median and interquartile ranges (IQR) were calculated when variables were not normally distributed. The proportion of patients depending on the duration from HIV diagnosis to onset of sleep disturbance was reported as percentages. Independent two-sample t-test were used to compare means and chi-square test or Fisher’s exact test were used to compare group differences. For non-parametric data, Mann-Whitney U-test was used. All statistical analyses were conducted using SPSS version 25 (IBM Corp., Armonk, NY, USA).
RESULTS
Through the SCRAP service, we found that 674 patients were first diagnosed with HIV/AIDS from January 1, 2012 to December 31, 2021. Of the 674 HIV-infected patients, 56 (8.3%) experienced sleep disturbances at least once. Among 56 patients, 48 had ICD-10 codes including F51.0, G47.0, and G47.3, and 8 patients prescribed hypnotic drugs only without an ICD-10 diagnosis. All those prescribed medication was received zolpidem, one of which was received triazolam as well.
The demographic and clinical characteristics of patients at the time of HIV diagnosis or at the onset of sleep disturbance are reported in Table 1. A total of 6 patients had experiences of psychiatric treatment before HIV diagnosis; 2 for alcohol use disorder, 2 for bipolar disorder, 1 for depressive disorder, and 1 for psychotic disorder. Seventeen out of 56 patients were provided psychiatric consultations after HIV diagnosis; 9 for insomnia, 3 for anxiety, 3 for insomnia and anxiety, 1 for depression, and 1 for delirium. Of the 17 patients, 8 patients (47.1%) visited psychiatric outpatient clinic at least once.
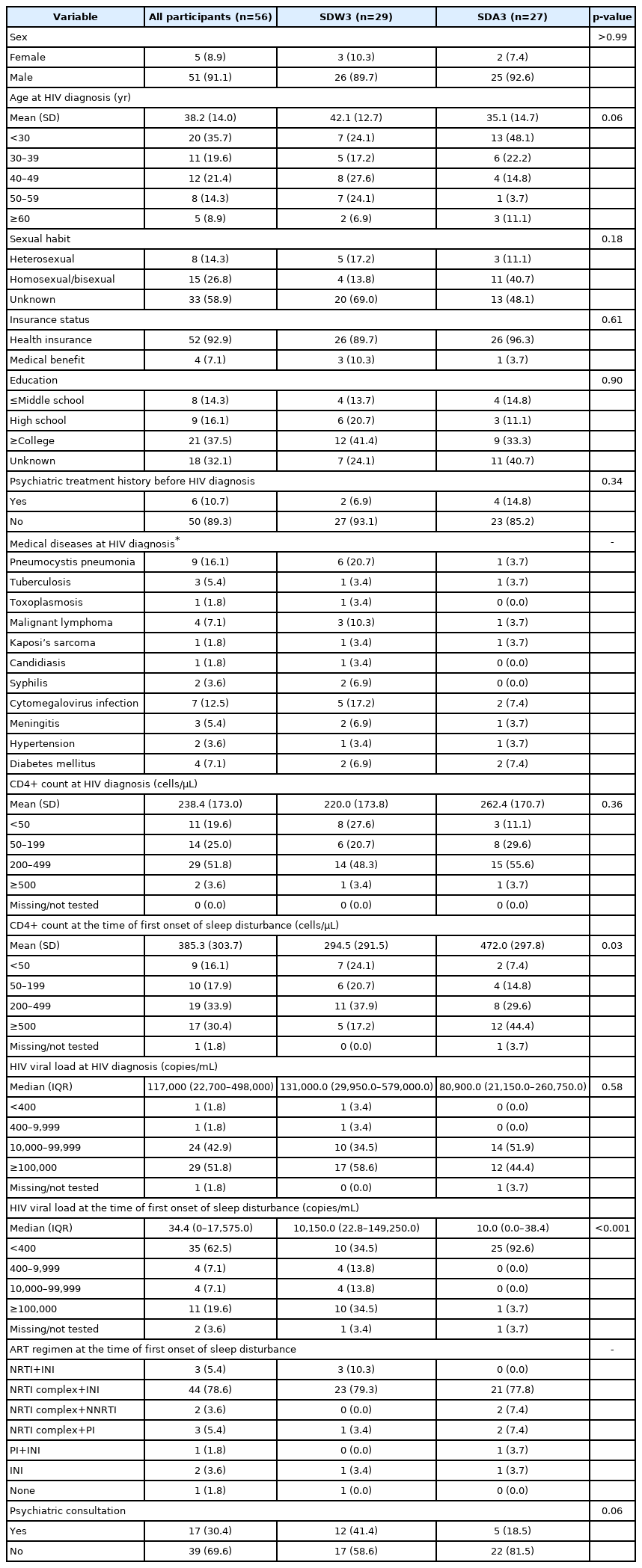
Demographic and clinical characteristics among two groups based on the onset time of sleep disturbance after HIV diagnosis
Regarding CD4+ cell count, the mean CD4+ T cell counts at HIV diagnosis was 238.4 cells/µL (SD, 173.0) and increased to 385.3 cells/µL (SD, 303.7) at the time of the first onset of sleep disturbance. The median HIV viral load was decreased from 117,000 copies/mL (IQR, 22,700–498,000) at HIV diagnosis to 34.4 copies/mL (IQR, 0–17,575) at the time of the first onset of sleep disturbance.
Given that majority of patients have experienced sleep disturbance within 3 months after HIV diagnosis (51.8%) (Figure 1), patients were divided into two groups based on the onset time of sleep disturbance after HIV diagnosis; the SDW3 group comprised 29 patients and the SDA3 group comprised 27 patients. Two groups were compared and results are reported in Table 1. No significant group differences were found in demographic variables. Regarding clinical variables, CD4+ cell count at the time of first onset of sleep disturbance was significantly lower in SDW3 group (p=0.03) and HIV viral load at the time of first onset of sleep disturbance was significantly higher in SDW3 group (p<0.001). There were no significant group differences in CD4+ cell count and HIV viral load at HIV diagnosis. The most common opportunistic infections were pneumocystis pneumonia (PCP) (20.7%), followed by cytomegalovirus (CMV) infection (17.2%), malignant lymphoma (10.3%), and syphilis (6.9%) in SDW3 group at the time of HIV diagnosis, which were higher than those of patients in SDA3 group. Regarding ART regimen, about 80% of patients in SDW3 group were receiving nucleoside reverse transcriptase inhibitor (NRTI) complex and integrase inhibitor (INI) based regimen at the time of first onset of sleep disturbance (79.3%). Of 29 patients in SDW3 group, 12 (41.4%) were provided psychiatric consultations. At the time of the first onset of sleep disturbances, 14 (48.3%) were receiving inpatient care in SDW3 group.
DISCUSSION
This study investigated clinical characteristics of PLWH with sleep disturbances after HIV diagnosis and potential risk factors of sleep disturbance according to the duration from HIV diagnosis to the onset of sleep disturbance by retrospective chart review. To the best of our knowledge, there were no published paper focusing on sleep disturbances among PLWH in South Korea.
Our findings showed that 8.3% experienced sleep disturbances in PLWH at a tertiary hospital over the past 10 years. Considering the higher prevalence reported in the previous studies (see the meta-analysis by Wu et al., 2015 [2]), this result suggests the possibility of underestimation and under-recognition of the actual occurrence of sleep disturbances in this population. The one probable explanation for under-recognition and underestimation is that the hypnotic medications prescribed at other clinics could not be detected and reported on electronic medical record unless the patients provide information. Otherwise, considering sleep disturbance insignificant compared with other complications of HIV infection or regarding it as a normal sequence of the disease might be the possible reasons for under-recognition and underestimation [19].
Of 56 patients, 17 patients (30.4%) were provided psychiatric consultation and 10 patients led to psychiatric outpatient treatment. The previous finding that 17.8% of HIV/AIDS inpatients referred for psychiatric consultation at a general hospital in South Korea supports our finding [20]. These results indicate that the significance of mental health issues on HIV patients might be underestimated probably due to the lack of attention and education in both physicians and patients. In addition, patients might be reluctant to disclose their HIV status and related psychiatric symptoms to psychiatrists due to the shame and fear of blame and rejection from other people [21].
More than half of patients (51.8%) were found to experience sleep disturbance in the first 3 months after HIV diagnosis. This finding is in line with the results of previous studies which reported the higher risk of sleep disturbances in the first several months after HIV diagnosis [1,22]. The findings that lower CD4+ T cell counts and higher HIV viral load at the time of first onset of sleep disturbance after HIV diagnosis in the SDW3 group could be associated with the results of opportunistic infections at the time of HIV diagnosis. The patients in SDW3 group had higher rates of PCP (20.7%), CMV infection (17.2%), malignant lymphoma (10.3%), and syphilis (6.9%) at the time of HIV diagnosis compared to those in SDA3 group. The results of lower CD4+ T cell counts and higher HIV viral load in SDW3 group might contribute to increased vulnerability to opportunistic infections and various symptoms including pain, fever, night sweat, cough, and dyspnea caused by opportunistic infections [23] might lead to sleep disturbances. Given that patients in the advanced stages of HIV are prone to opportunistic infections [24], these findings also suggests the possibility of late diagnoses with disease progression. However, reports on the association between CD4+ T cell counts and sleep disturbances are inconsistent. Some studies have reported that sleep disturbances among PLWH were related to lower CD4+ cell counts [22,25,26] while others have shown opposite finding [27] or no relationship [17,28,29]. Despite the role of standard primary markers of HIV exacerbation and treatment response, CD4+ cell counts and HIV viral load may not provide a whole picture of an patient’s immune health [13]. Thus, further studies of markers of immune activation or cytokines contributing to the pathophysiology of sleep disturbance should be needed.
Given that quality and quantity of sleep in hospitalized patients were negatively related to hospital-related factors including light and noise disturbances and awakenings by medical staff at night [30], our finding of 48.3% of hospitalized patients at the time of the first onset of sleep disturbance suggests another possible reason for sleep disturbance in early HIV infection. Furthermore, regarding that 12 out of total 17 cases were requested for psychiatric consultations in patients with shorter duration of HIV, greater perceived stress due to cognitions of being stigmatized [27] and psychiatric symptoms such as depression or anxiety should be considered as one of the possible reasons for early onset of sleep disturbances.
Our study had several limitations. First, regarding the data extracted from existing medical records, this study is prone to missing data. Second, participants were considered to experience sleep disturbance based on a history of being prescribed sleeping pills or having insomnia-related ICD-10 codes, rather than standardized instruments for sleep disturbances. Third, the presence of sleep disturbances may have been underestimated by excluding medications such as benzodiazepine, antidepressants, antihistamines, and melatonin which are often used to improve sleep disturbances. Fourth, since this is a descriptive study and not a hypothesis test, it was impossible to determine sample size in advance, and the sample size was also small. Finally, participants in this study were restricted to a single center, which may not be representative of the broader HIV-infected populations; therefore, researches investigating broader range of population are warranted.
In conclusion, this study provided data on potential clinical parameters related to sleep disturbances in PLWH. Regarding high prevalence of sleep disturbance in PLWH, our finding of 8.3% suggests the possibility of underestimation and under-recognition. Approximately 50% of patients have experienced sleep disturbance in the first 3 months after HIV diagnosis, which might be possibly related to higher rates of opportunistic infections caused by lower CD4+ T cell counts and higher HIV viral load at the time of first onset of sleep disturbance. For early detection and diagnosis of sleep disturbance in PLWH, further elucidating the underlying pathophysiology of sleep disturbance and association with immune activation is warranted in future studies.
Notes
Funding Statement
The National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning, Republic of Korea, supported the present study (Grant number: 2022R1A2B5B03002611). The funding source was not involved in the study design, collection of data, or writing of the report.
The authors have no potential conflicts of interest to disclose.
Availability of Data and Material
All data used during this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Author Contributions
Conceptualization: all authors. Funding acquisition: Eun Lee. Investigation: all authors. Supervision: Eun Lee. Writing—original draft: Suonaa Lee, Eun Lee. Writing—review & editing: Suonaa Lee, Eun Lee.