 |
 |
- Search
| Chronobiol Med > Volume 5(3); 2023 > Article |
|
Abstract
Objective
Methods
Results
Conclusion
NOTES
Funding Statement
This study was funded by Kuhnil Pharmaceutical Co., Ltd. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
Availability of Data and Material
The datasets generated or analyzed during the study are not publicly available due to the privacy or ethical restrictions but are available from the corresponding author on reasonable request.
Author Contributions
Conceptualization: Young-Min Park. Data curation: all authors. Formal analysis: all authors. Funding acquisition: all authors. Investigation: all authors. Methodology: Young-Min Park. Project administration: Young-Min Park. Resources: Young-Min Park. Software: Young-Min Park. Supervision: Young-Min Park. Validation: all authors. Visualization: all authors. Writing—original draft: all authors. Writing—review & editing: all authors.
Figure 1.
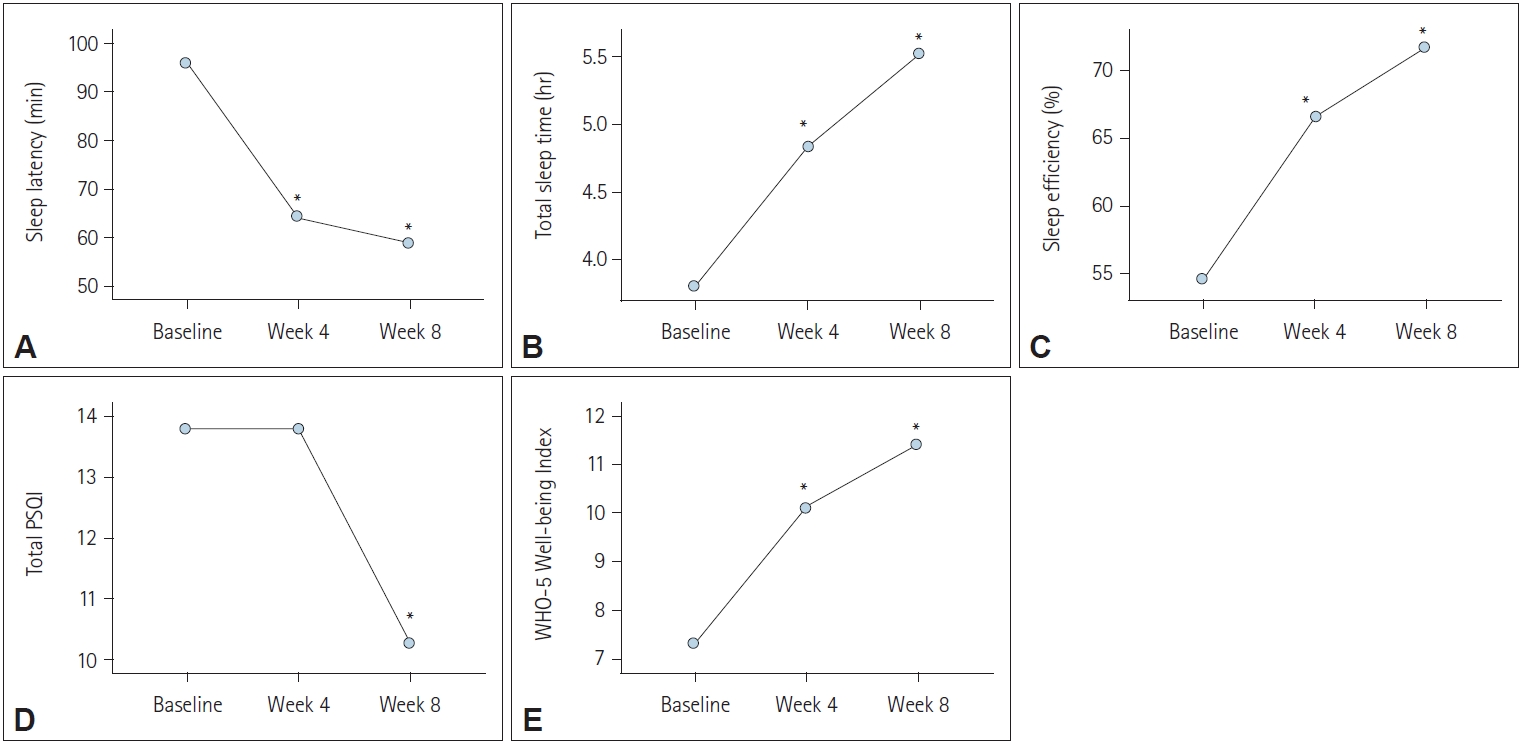
Figure 2.
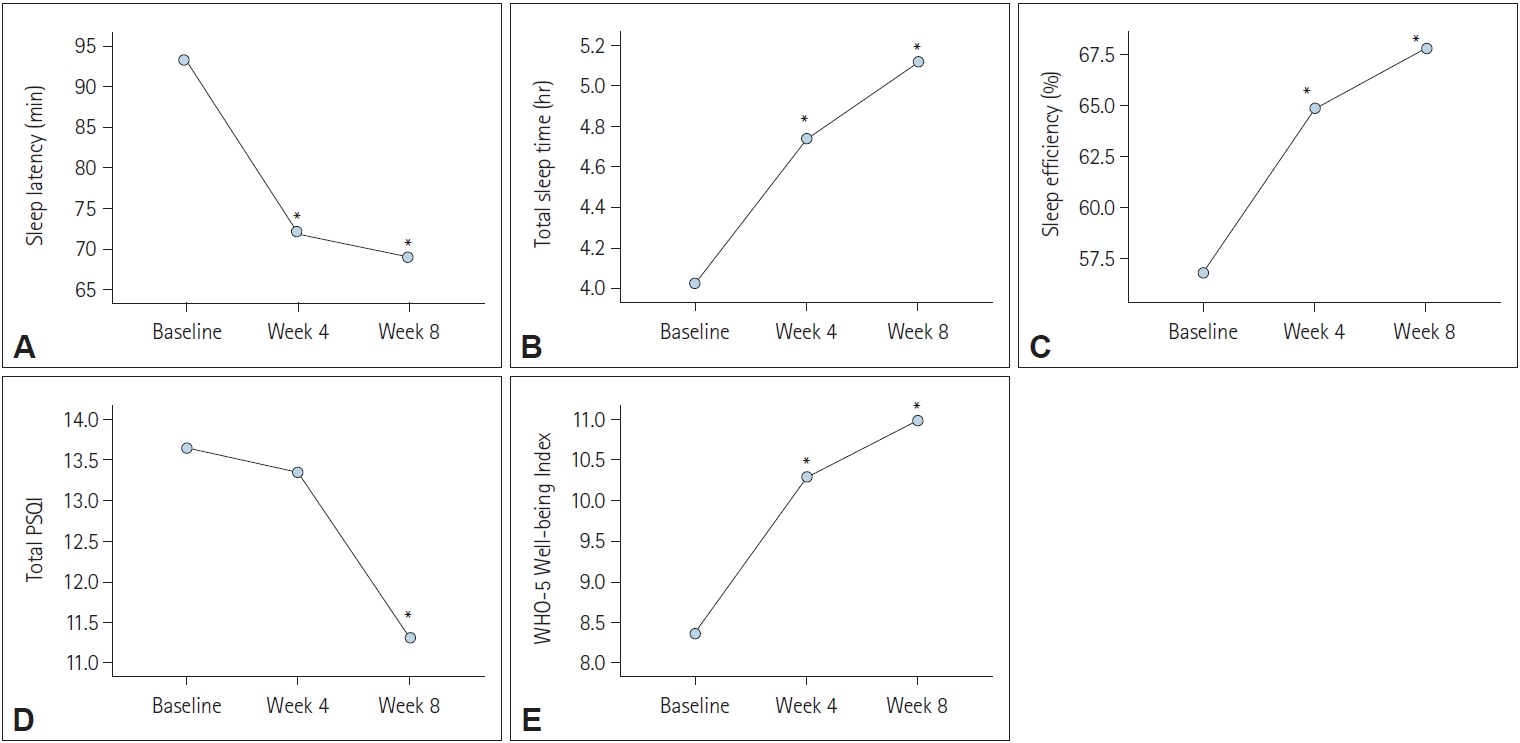
Table 1.
| Variables | Baseline | Week 4 | Week 8 | F, p-value* | Post-hoc |
|---|---|---|---|---|---|
| Sleep latency (min) | 94.70±66.78 | 64.09±68.44 | 57.27±81.62 | 10.45, p<0.001 | Baseline>week 4, week 8 |
| Total sleep time (hr) | 3.92±2.00 | 4.93±1.83 | 5.61±2.04 | 27.21, p<0.001 | Baseline<week 4<week 8 |
| Sleep efficiency (%) | 55.14±22.59 | 66.94±21.06 | 72.32±18.30 | 21.16, p<0.001 | Baseline<week 4<week 8 |
| Total PSQI | 13.94±2.98 | 13.85±3.23 | 10.36±4.05 | 32.29, p<0.001 | Baseline, week 4>week 8 |
| WHO Well-being Index | 7.15±5.38 | 9.74±5.62 | 10.91±5.87 | 20.81, p<0.001 | Baseline<week 4<week 8 |
Table 2.
| Variables | Baseline | Week 4 | Week 8 | F, p-value* | Post-hoc |
|---|---|---|---|---|---|
| Sleep latency (min) | 93.21±72.99 | 72.90±73.80 | 69.00±82.26 | 11.28, p<0.001 | Baseline>Week 4, Week 8 |
| Total sleep time (hr) | 4.03±1.85 | 4.73±1.78 | 5.11±1.98 | 22.30, p<0.001 | Baseline<Week 4<Week 8 |
| Sleep efficiency (%) | 56.82±23.34 | 64.83±22.80 | 67.79±21.83 | 17.48, p<0.001 | Baseline<Week 4<Week 8 |
| Total PSQI | 13.65±3.15 | 13.34±3.45 | 11.32±4.02 | 25.73, p<0.001 | Baseline, Week 4>Week 8 |
| WHO Well-being Index | 8.38±5.46 | 10.29±5.42 | 10.98±5.52 | 15.29, p<0.001 | Baseline<Week 4<Week 8 |







