|
 |
- Search
| Chronobiol Med > Volume 2(3); 2020 > Article |
|
Abstract
The hormone of darkness ŌĆ£melatoninŌĆØ has shown extraordinary potential. Owing to versatile properties which include, but are not limited to, strong antioxidation, anti-inflammatory, anti-apoptotic, and chronotherapeutic action has generated a great deal of interest as a therapeutic drug for various diseases especially neural damage and neurodegenerative diseases. Melatonin has shown to be effective in arresting neurodegeneration in experimental models of AlzheimerŌĆÖs, HuntingtonŌĆÖs, and ParkinsonŌĆÖs disease, subarachnoid hemorrhage, and ischemic stroke. In this article, the authors review the role of melatonin in neural damage and associated diseases. Briefly, we will discuss the function and protective role of melatonin in the central and peripheral nervous systems. Subsequently, an overview of the role of melatonin in endoplasmic reticulum and mitochondria in neural tissues along with possible biological pathways will be addressed. Finally, we will review the action of melatonin on programmed cell death and its probable molecular mechanism.
Melatonin (N-acetyl-5-methoxytryptamine), commonly known as the sleep hormone or hormone of darkness, is a phylogenetically conserved molecule with numerous versatile and diverse functions [1-4]. In general, melatonin has; 1) antioxidant activity primarily by its free radical scavenging, 2) antiapoptotic property mostly by blocking caspase-3 cleavage and mitochondrial permeability transition pore opening, 3) anti-inflammatory action generally by inhibiting inflammasome activation, and 4) chronobiotic abilities by acting as a sleep inducer [5-11]. Moreover, in different organisms, the function of melatonin includes maintenance of gut motility, circadian and sedation (sleep) activity, immune modulation, osmoregulation, shoaling and schooling, locomotor activity, regulation of cell cycle, food intake, thermal preference management, skin pigmentation, reproduction and growth [12-17]. This multifunctional and multipotent indolamine is primarily produced in the pineal gland in a circadian manner synchronized with photoperiodic information received via the retinohypothalamic pathway [18]. Recent studies have proved that melatonin can be synthesized from several extra pineal sites like retina, brain, gut, testis, and ovary [4,19-22]. The presence of melatonin receptors in almost all of the vertebrate tissues is reported, which is an indicator of the functional prowess of melatonin [22-26]. Melatonin is not a conventional hormone as it can functions via receptor-dependent and receptor-independent manner by binding with nuclear binding sites or serine/threonine (Ser/Thr) kinases like death-associated protein kinase 1 [27-30]. Additionally, melatonin is amphiphilic, this character of melatonin provides it the ability to diffuse and cross all morpho-physiological barriers, enter all the cells, and influence numerous function [31-37]. Owing to the virtual omnipresence, cytoprotective, and chronobiotic abilities along with biocompatibility, melatonin is considered as a powerful therapeutic drug for the treatment of various diseases (Figure 1). As melatonin can cross-blood brain barrier and enter any cell and modulate many functions, melatonin is used as a drug for many degenerative neural damages such as AlzheimerŌĆÖs disease (AD), ParkinsonŌĆÖs disease (PD), HuntingtonŌĆÖs disease (HD) or amyotrophic lateral sclerosis, etc.
Progressive degradation of the neurons in the sensory, motor, or cognitive system is the primary characteristic of neurodegenerative disorders leading to diseases such as AD, dementia, PD, or HD. Neurodegeneration in all these disorders follows similar molecular processes like oxidation mediated degeneration, impairment of mitochondria, excitotoxicity, and inflammation [38,39]. Even though the regular intake of drugs with antioxidants, antiinflammatory, and antiapoptotic activity has been proposed for management or treatment of neural damage, however, their effectiveness has been questioned and most of them are under the primary stages of drug development (Table 1) [40-69]. In this context, melatonin simultaneously offers antioxidant, anti-inflammatory, and antiapoptotic activity. Therefore, melatonin is considered as a promising target for the treatment or management of neurodegenerative diseases. The focus of this article is on the role of melatonin in neural tissues. We will discuss the role of melatonin in the central nervous system (CNS) and peripheral nervous system (PNS). Following that, an overview of functions of melatonin in the endoplasmic reticulum (ER) and mitochondria in neural tissues will be addressed. Finally, we will review the role of melatonin on apoptotic cell death and autophagy and its associated pathway.
Neural tissues, located in both the CNS and PNS, exhibit degenerative changes with age. Even though the formation of new myelin has been reported to occur, myelin sheaths with dense cytoplasm and balloons have also been observed [70]. As these occurrences affect functions such as conduction velocity and normal timing in the neural circuit, structural changes of myelin lead to cognitive decline with age [70]. Furthermore, neural degeneration in the PNS is induced by detrimental stimuli or injury [71,72]. Peripheral nerve injury typically results in the loss of sensory, motor, and autonomic functions either partially or totally. Damage induced by peripheral nerve injury occurs from the original lesioned nerves to the denervated segments of the body due to the discontinuity of axons, degeneration of nerve fibers, and eventual death of injured neurons [73]. Previous studies have found increased oxidative stress in the neural tissues of subjects from an AD animal model [74-77]. Reactive oxygen species (ROS) are primarily generated by the Fenton reaction, which induces intracellular mitogen-activated protein kinase (MAPK) activation, Ca2+ dyshomeostasis, and apoptosis [78,79]. Interestingly, the oxidative condition is correlated with the clinical symptoms of AD such as memory deficits and cognitive impairments in both rodents and humans [80,81]. Similarly, sciatic nerve crush injury is a well-established rodent model due to its ability to impair axonal connectivity [82]. There is an up-regulation of oxidative stress-induced lipid peroxidation as well as a down-regulation of glutathione (GSH) and catalase in the sciatic nerve constriction model [83]. Cumulatively, ROS generation is induced when nerves are injured in both CNS and PNS. Therefore, researchers have focused on potent antioxidant substances that inhibit the progression of AD. Numerous studies have demonstrated the antioxidant effects of natural products or chemicals in vitro and in vivo and have shown that decreased oxidative stress improves neurological function in rodents with A╬▓-induced AD and humans [80,84-87]. Olcese et al. [88] observed a beneficial effect of melatonin regarding the prevention of cognitive deficits and the suppression of oxidative stress-induced neurodegeneration via the up-regulation of antioxidant enzymes [e.g., superoxide dismutase (SOD-1), glutathione peroxidase, and catalase] and enhancement of the anti-inflammatory response by down-regulation of tumor necrosis factor-alpha (TNF╬▒). Recently, the regulatory functions of melatonin, which are involved in its neuroprotective action against A╬▓-induced neurotoxicity, were identified. It was shown that the neuroprotective effects under A╬▓-induced toxicity are mediated by the ROS scavenging and the protection of astrocytes from mitochondrial depolarization [89]. In addition to its antioxidant properties, melatonin produces anti-inflammatory and antiapoptotic activity and increases neurotrophin production during AD progression both in vitro and in vivo [90-94]. These studies performed with AD patients have found a significant association between melatonin administration and improved sleep patterns [95]. Therefore, it may be beneficial to focus not only on the role of melatonin during primary neuropathological progression but also on secondary AD-induced problems such as the interruption of sleep. Shokouhi et al. [96] found that melatonin reduces lipid peroxidation in sciatic nerve-injured animals in a dose-dependent manner. Also, in the sciatic nerve transection model, treatment with melatonin reduces motor neuron loss in the lumbar spinal cord, stimulates SOD1/2 production, and aids in the recovery of action potentials in the crushed sciatic nerve both in vitro and in vivo [97,98]. Melatonin also inhibits collagen deposition in the neuroma formed by the injured sciatic nerve, whereas melatonin depletion, as induced by pinealectomy, increases collagen content [99]. Moreover, compared to control, melatonin treatment elevates the sciatic functional index, improves conduction velocity, and attenuates latency values as measured by in vivo electromyography; with no difference between low (5 mg/kg) and high (20 mg/kg) doses [100]. Therefore, it can be concluded that melatonin can stimulate neural regeneration through a decrease of pro-oxidants and the up-regulation of neurotrophic factors.
The ER has important functions, which include the synthesis, folding, and translational modification of proteins. ER stress occurs when ER homeostasis is abnormally regulated and results in the activation of unfolded protein responses (UPR) to reduce ER stress [101]. UPRs are initiated by three ER membrane-associated proteins, PRKR-like endoplasmic reticulum kinase (PERK), inositol requiring enzyme 1 (IRE), and activating transcription factor 6 (ATF6╬▒), which up-regulate the expression of ER chaperones such as binding immunoglobulin protein/glucose-regulated protein 78 and 94 (Bip/Grp78 and Grp94) [102]. The accumulation of misfolded proteins, which activate UPRs, is a common feature of many neurodegenerative diseases, including AD, PD, and HD [103-107]. These studies indicate the importance of ER homeostasis and its role in the degenerative changes of neural tissues.
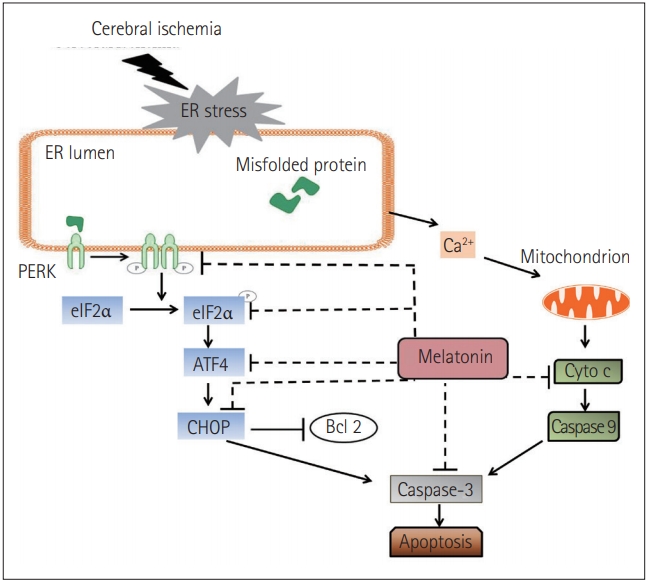
Interestingly, there are AD-like phenotypes, including spatial memory impairment, tau hyperphosphorylation, and the up-regulation of ER chaperones (e.g., BiP/GRP78, CHOP/GADD153), in illumination-induced melatonin-deprived rats [108]. Brain damage induced by Arsenite exposure increases ATF4, C/EBP homologous protein (CHOP), sXBP1, and pro-caspase 12 levels whereas, melatonin represses the expressions of these factors and leads to an inhibition of apoptosis [109]. In another report, Kang et al. [110] found that treadmill exercise suppresses ER stress responses via the inhibition of PERK, eukaryotic translation initiation factor 2╬▒ (eIF2╬▒), ATF6╬▒, sXBP1, and CHOP activation, reduces the deposition of A╬▓-peptide42, and prevents memory dysfunction in aged PS2 mutant mice. Recently, it has been shown that in cerebral ischemia mice, melatonin operates with the same pathway. It not only decreases the phosphorylation of PERK and eIF2╬▒ but also suppresses the expression of ATF4 and CHOP. Simultaneously, the level of cytochrome c (Cyto c) was reduced and the expression of caspase-3 was down-regulated (Figure 2) [111]. Based on the aforementioned evidence, it can be concluded that melatonin contributes to the amelioration of neural degeneration through ER-associated signaling pathways.
A growing amount of evidence indicates that mitochondria play a pivotal role in the pathophysiology of various diseases [112]. Mitochondrial damage can occur through the impairment of electron flux via the electron transport chain, which results in electron leakage and generates oxidation in a self-stimulatory feedback loop. Recent studies have shown that melatonin can attenuate the generation of ROS and reactive nitrogen species (RNS) from mitochondria under several detrimental conditions and protect against the oxidative, nitrosative, and nitrative damage of electron transport chain proteins. Additionally, melatonin prevents lipid peroxidation in the inner membrane of mitochondria, which leads to electron flux and energy efficiency [113-115]. To understand the mechanisms underlying these findings, the site of radical generation in mitochondria and the causes of mitochondrial dysfunction should be addressed.
Melatonin has been shown to negatively modulate free radicals, and the regulation of inducible nitric oxide (NO) synthase (iNOS) and neuronal NO synthase (nNOS) [116]. Inflammatory and stressassociated signaling primarily up-regulates iNOS in astrocytes, microglia, and macrophages. In contrast, excitation dependent Ca2+ influx affects the regulation of nNOS, especially in glutamatergic neurons. Although a reasonable level of increase in NO radicals is likely to be suitable for mitochondrial function, significantly high levels often induce severe impairments in the electron transfer chain (ETC) [117]. The adverse effect of the NO radical and its non-enzymatically formed metabolites are responsible for the impairment of ETC, especially under detrimental conditions such as chronic inflammation [118]. Melatonin has been reported to have a potent influence on carbonate radicals and to be a scavenger of hydroxyl radicals [119-122]. Furthermore, the nitrosation of armates has been observed in the nitrosodioxyl radical, an analog of peroxynitrite that could be formed by electron abstraction from peroxynitrite; however, this has not been studied in electron transport chain proteins. If melatonin is administrated at high pharmacological doses, it might react with products mostly originating from these peroxynitrite. Nonetheless, at low pharmacological concentrations, the potential to inhibit the up-regulated iNOS or nNOS exists. This action of melatonin can break the malicious cycle of high NO radicals, which leads to a blockade of the electron transport chain in various ways, including by combining with the superoxide anion to produce strongly reactive factors that impair respirasomes, interrupt electron flux, and induce electron backflow and leakage.
Mitochondrion-dependent apoptosis, also termed the intrinsic apoptotic pathway, is one of the major apoptotic signaling pathways [123]. Mitochondrion-dependent apoptosis causes mitochondrial membrane permeabilization (MMP), caused by the opening of mitochondrial permeability transition pore (mPTP), which refers to the swelling and depolarization of mitochondria evident upon the development of a cytosolic calcium burst or when oxidative stress is in play [124-126]. MMP is thought to block ATP synthesis by oxidative phosphorylation in mitochondria and to stimulate ATP hydrolysis [125]. Melatonin levels have been reported to be higher inside the mitochondria than in the plasma [127]. Moreover, melatonin is thought to contribute to mitochondrial homeostasis [128,129] by 1) facilitating hydrogen peroxide (H2O2) scavenging (H2O2 is one of the most important ROS produced by mitochondria) and 2) improving mitochondrial function (increasing ATP synthesis, reducing ROS production, and protecting against abnormal decreases in mitochondrial membrane potential which could, in turn, trigger MMP) [130,131]. The release of Cyto c from mitochondria into the cytosol can initiate mitochondrion-dependent apoptosis via downstream activation of cell death pathways [132]. Melatonin not only blocks mPTP opening but also the mPTP-dependent release of Cyto c, whose release would inhibit caspase 3, triggering apoptotic cell death [133]. Some authors consider that ROS generation, caused by a decrease in mitochondrial membrane potential, may trigger mitochondrion-dependent apoptosis [128,134]. Similarly, the opening of mPTP under oxidative stress may play a role in the induction of apoptosis [128]. Melatonin maintains mitochondrial membrane permeability by preventing lipid peroxidation and also controls mitochondrial H2O2 levels, both under normal conditions and when oxidative stress is in play [11,128]. Additionally, melatonin seems to inhibit the ROS-induced increase in calcium levels [135]. Oxidative stress is a powerful regulator of the sensitivity of mPTPs to calcium ions. Thus, melatonin indirectly blocks the opening of the mPTPs and the induction of apoptotic cascades via the release of mitochondrial Cyto c and activation of caspase 3 [11].
Endogenous levels or exogenous administration of melatonin offers protection to injured cells from several cell death forms like, mPTP-driven cell death, necroptosis, apoptosis, and autophagy. Melatonin is highly permeable in a variety of cells organelles too, and its protective effects can be mediated through both melatonin receptor-dependent or independent manner. Inside the cytoplasm, melatonin inhibits the receptor-interacting serine/threonine-protein kinase 3 (Ripk3) mechanisms, up-regulation of dynamin-related protein (Drp1), and Bax-dependent Cyto c release caused by external insults, leading to pro-survival signals. Mitochondrial melatonin offers protective roles by interaction with peptide transporter 1 (PEPT1/2) and Glucose transporter (GLUT), which are considered as the novel melatonin receptors in the mitochondrion (Figure 3) [11].
Programmed cell death (PCD), refers to cell death mediated by intracellular programs. Three forms of PCD are known: apoptosis, autophagy, and programmed necrosis, apoptosis is most well understood [136]. PCD is important during neural development as it plays a central role in rendering the neuronal environment by regulating both the numbers and types of cells in the CNS [137,138]. Apoptosis is critical in regulating neuronal cell death, both during neural development and in various neurodegenerative diseases. Lower expression of apoptotic protease activating factor 1 (Apaf1) expression, can lead to abnormal brain enlargement because of apoptosis deregulation and may cause prenatal fatality in experimental animals [139,140]. Some studies have shown that excessive apoptotic cell death attributed to the lack of the antiapoptotic protein Bcl-x may cause neuronal immaturity in the developing brain and spinal cord leading to embryonic death [141]. Moreover, it is also shown that primary spinal cord injury is generally followed by apoptosis, inflammation, free-radical-induced lipid peroxidation, and vascular damage [142,143].
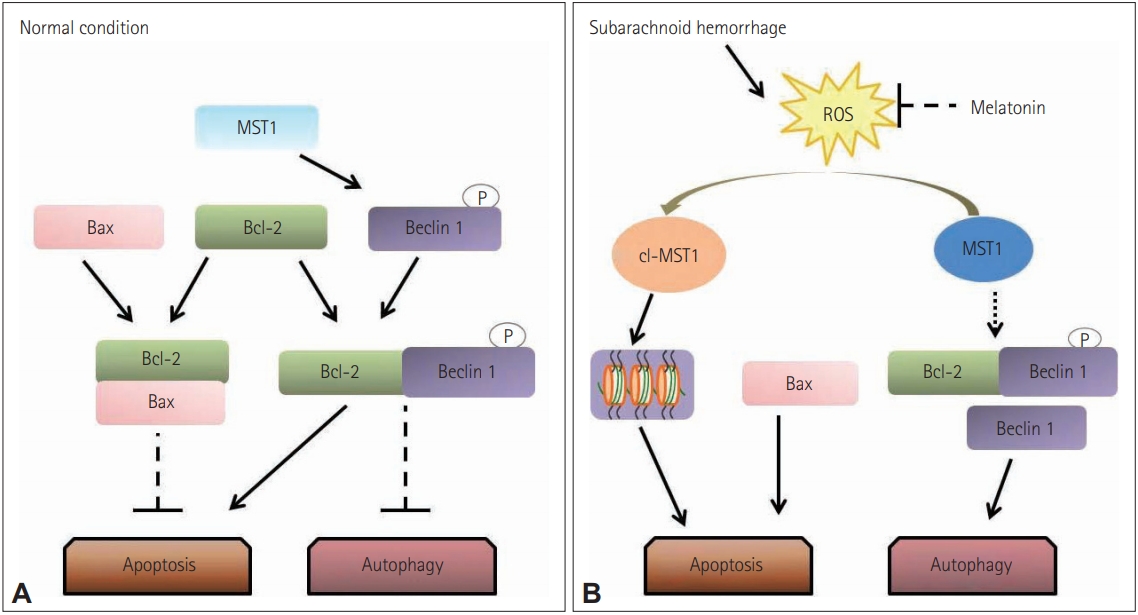
A considerable amount of literature has shown that apoptotic cell death can be affected by melatonin. Neural injuries elicit excitotoxicity, alteration of calcium ion homeostasis, increased nitric oxide synthesis, and finally, apoptosis [30,144]. Melatonin seems to protect neuronal cells from apoptotic cell death-mediated neurotoxicity by regulating the expression of proteins by enhancement of the interaction between pBad and 14-3-3, inhibiting activation of apoptosis cascades [145,146]. In subarachnoid hemorrhage (SAH), exogenous melatonin can reduce SAH-induced early brain injury by inhibiting excessive neuronal apoptosis and autophagy probably by the reactive oxygen speciesŌĆōmammalian sterile 20-like kinase 1 (ROS-MST1) pathway (Figure 4) [147].
Autophagy, another type of PCD, is critical as it balances the formation and degradation of proteins, therefore, autophagy is not only a cell death pathway, but it can also promote cell survival [138,148]. Recently, autophagy has been considered as a pro-survival signaling pathway as it has shown a protective effect against necrotic cell death in neonatal rats subjected to brain damage by hypoxia-ischemia [149]. Conversely, deleterious aspects of autophagy have also been reported. After middle cerebral artery occlusion, the size of infarct was reduced upon injection of an inhibitor of autophagy [150]. Additionally, both brain edema and total infarct volume were also reduced by such an autophagy inhibitor [151]. After traumatic brain injury, autophagy is thought to induce cell death. Consequently, the effect of autophagy on the fate of cells remains ambiguous.
It has recently been shown that melatonin regulates autophagy. Park et al. [152] have shown melatonin induced rapid activation of autophagy after spinal cord injury. Inversely, Guo et al. [153] suggested that inhibition of autophagy could eliminate any beneficial role played by melatonin. The same study also reported the neuroprotective role of autophagy was augmented by melatonin, indicating that activation of autophagy might be protective [153]. To explain the Janus-faced nature of autophagy, it was suggested that melatonin-mediated autophagy induces autophagic cell death only if the extent of cellular damage exceeds a certain level, whereas it promotes cell survival if the damage is less extensive [153]. Conversely, other researchers have found that melatonin can be anti-autophagic. It has been proposed that melatonin plays a protective role after brain injury by activating the Class I Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway, which negatively regulates autophagy [154]. Similarly, it has been suggested that melatonin exerts beneficial effects after brain injury by activating the rapamycin kinase pathway (mTOR/p70S6 kinase), the downstream target of Akt [30]. Activation of autophagy by inhibiting apoptosis can be beneficial in several circumstances. Such activation improves locomotor function in animals with spinal cord injuries [155]. A tumor-suppressing effect was also evident [74], as was the melatonin-induced improvement of cell survival in animal models of SAH [156,157]. Interestingly, the simultaneous down-regulation of apoptosis and autophagy may exert protective effects in animal models of brain injury [158]. Future studies should explore the details of the complex interactions between the mechanisms of PCD and neural injury.
Oxidative stress and inflammatory response leading to different modes of PCD have been involved in the pathogenesis of various neurodegenerative diseases such as AD, PD, HD and other neurological conditions including stroke, SAH, SCI, and brain trauma. Generally, high oxygen utilization and low anti-antioxidant levels are the main reasons for enhanced oxidative stress in damaged neural tissue and aged individuals. Melatonin is an excellent drug both in vivo and in vitro in neurodegenerative diseases. Melatonin has been shown to reduce derogatory neuropathological changes not only by ROS and RNS scavenging but also by decreasing the phosphorylation of PERK/eIF2╬▒ and suppressing ATF4/CHOP which down-regulates the caspase-3 mediated apoptosis. Furthermore, melatonin may also inhibit excessive neuronal apoptosis and autophagy, possibly by the ROS-MST1 pathway. The fact that melatonin easily crosses the blood-brain barrier, enters any cell, acts with and without the receptor, along with its high tolerance and biocompatibility even at the highest dosages, represents the significance of melatonin in treatment or management of neural damage. However, the number of randomized controlled studies testing the efficacy of melatonin on neural damage and neurodegenerative disorders is limited, and the experiment quality is often unsatisfactory. Lack of potential biomarkers to study the changes in neural damages after drug treatment makes these studies more challenging. There is a need for more clinical trials to study the protective role of melatonin. Moreover, the search for reliable biomarkers to study the post-drug administration changes in neural damage should be expedited.
Acknowledgments
The authors would like to acknowledge the invaluable support and critical comments of members in ŌĆśBiological Clock & Aging ControlŌĆÖ laboratory. This work was supported by a grant from the National Research Foundation (NRF-2020R1A2C201215511), Korea. Jeonghyun Choi is supported by a post-doctoral fellowship from National Research Foundation (NRF-2019R1A6A3A01091422), Korea.
NOTES
Conflicts of Interest
Author Contributions
Conceptualization: Yonggeun Hong, Yunkyung Hong, Zeeshan Ahmad Khan. Data curation: Zeeshan Ahmad Khan, Yunkyung Hong, Jeonghyun Choi, Youngjeon Lee, Yunho Jin. Project administration: Yonggeun Hong. Resources: Yonggeun Hong. Supervision: Yonggeun Hong. WritingŌĆöoriginal draft: Zeeshan Ahmad Khan, Yunkyung Hong, Yonggeun Hong. WritingŌĆöreview & editing: Yonggeun Hong, Zeeshan Ahmad Khan.
Figure┬Ā2.
The mechanisms of melatonin protected neurons against endoplasmic reticulum (ER)-induced apoptosis after cerebral ischemia. Reprinted from Lin et al. Int J Mol Med 2018;42:182-192 [111], according to the Creative Commons License (CC-BYNC-ND).
Figure┬Ā3.
Antiapoptotic mechanisms operated by melatonin. Reprinted from Tarocco et al. Cell Death Dis 2019;10:317 [11], according to the Creative Commons License (CC-BY).

Figure┬Ā4.
Proposed role of melatonin in regulating the balance between apoptosis and autophagy. (A) In normal situations, Bcl-2 binds with Bax and inhibiting the Bax-induced channel formation which is permeable to cytochrome c to activate caspase-3 dependent apoptosis. Additionally, MST1 present in the cell helps in cell survival by phosphorylating Beclin 1 which prevents the synthesis of Beclin 1-Atg14L-Vps34, which can inhibit autophagy and activate apoptosis. (B) In subarachnoid hemorrhage stress, the increased brain reactive oxygen species (ROS) content activates the cleavage of MST1 to produce cl-MST1, which is transferred into the nucleus and phosphorylates several histones, inducing neuronal cell apoptosis. Besides, subarachnoid hemorrhage reduces the level of Bcl-2 protein, displacing Bax from Bcl-2 to enhance apoptosis. In the meantime, both the down-regulation of Beclin 1 phosphorylation by MST1 and the low expression of Bcl-2 cause the dissociation of the Bcl-2/Beclin 1 complex, increasing the cell autophagy. Reprinted form Shi et al. Front Mol Neurosci 2018;11:93 [147], according to the Creative Commons License (CC-BY).
Table┬Ā1.
List of drugs for neurodegenerative diseases with the mode of action/target including antioxidant or anti-inflammatory or antiapoptotic activity
| Drug | Company | Highest dev. status | Indication | Mode of action/drug effect |
|---|---|---|---|---|
| AM-36 [40] | AMRAD Corp., Ltd. | Discovery | Cerebrovascular ischemia; AD; spinal cord injury | Antioxidant agent; sodium channel blocker; |
| Dehydroascorbic acid [41] | Memorial Sloan Kettering Cancer Center | Discovery | AD; ND; PD; cerebrovascular ischemia | Antioxidant agent |
| Imidazolyl nitrones [42,43] | Servier | Discovery | Nervous system injury; ND | Neuroprotectant; antioxidant agent |
| Mitoquinone/mitoquinol redox mixture [44] | Antipodean Pharmaceuticals, Inc. | Phase 2 | PD; sunburn; liver disease | Neuroprotectant; antiparkinsonian; antioxidant agent; anti-inflammatory |
| NAPVSIPQ [45] | National Institutes of Health | Discovery | ND | Antioxidant agent; neuroprotectant |
| NOX-700 [46] | Medinox, Inc. | Phase 1 clinical | ND | Antioxidant agent |
| PAN-811 [47] | Panacea Pharmaceuticals, Inc. | Discovery | AD | Antioxidant agent; neuroprotectant |
| Lipid soluble antioxidants [48] | OXIS International, Inc. | Discovery | AD; PD; cardiovascular disease; diabetes mellitus | Antioxidant agent; antiparkinsonian; neuroprotectant; cardioprotectant |
| S-33113-1 [48] | Servier | Discovery | ND; cerebrovascular ischemia | Antioxidant agent; neuroprotectant |
| QR-333 [49] | Quigley Pharma, Inc. | Phase 2 | Diabetic neuropathy | Antioxidant agent; aldose reductase inhibitor/neuroprotectant |
| AAD-2004 [50] | Neurotech Pharmaceuticals Co., Ltd./AmKor Pharma, Inc. | Discovery | AD; dementia; beta amyloid antagonist | Anti-inflammatory; antioxidant agent; neuroprotectant; antiparkinsonian |
| AEOL-10150 [51] | Aeolus Pharmaceuticals, Inc. | Phase 1 | Motor neurone disease; lung inflammation; poison intoxication | Anti-inflammatory; antioxidant; angiogenesis inhibitor; neuroprotectant; radioprotectant |
| AO-1-530 [52] | Antoxis, Ltd. | Discovery | Cerebrovascular ischemia | Antioxidant agent; free radical scavenger; neuroprotectant |
| Catalytic antioxidants [53] | Aeolus Pharmaceuticals, Inc. | Discovery | Chronic bronchitis; motor neuron disease; PD; asthma; cerebrovascular ischemia; cancer; epilepsy | Antioxidant agent; anticancer; radioprotectant; anticonvulsant agent |
| VX-799 [54,55] | Serono; Vertex Pharmaceuticals, Inc. | Discovery | ND; cardiovascular disease; cerebral infarction; cerebrovascular ischemia | Apoptosis inhibitor; neuroprotectant; vasoprotectant; anti-inflammatory |
| ReN-1820 [56] | ReNeuron (UK), Ltd. | Discovery | AD; inflammation; pain; ND; dementia | NGF antagonist; nootropic agent; analgesic; anti-inflammatory |
| AEG-3482 series [57] | Aegera Therapeutics, Inc. | Discovery | Multiple sclerosis; cerebrovascular ischemia; cancer | Apoptosis inhibitors; neuroprotectant |
| DP-109 [58] | D-Pharm, Ltd. | Discovery | ND | Chelating agent; apoptosis modulator; neuroprotectant |
| DP-b99 [59,60] | D-Pharm, Ltd./Yungjin Pharmaceutical Co., Ltd./Wanbang Biopharma | Phase 3 (phase 2) | Cerebrovascular ischemia; brain injury; ND | Anti-inflammatory; antioxidant agent; chelating agent; apoptosis modulator; neuroprotectant |
| Huperzine-A (transdermal patch, AlzheimerŌĆÖs disease) [61] | Neuro-Hitech, Inc./Xel Herbaceuticals, Inc. | Phase 1 | AD; acetylcholinesterase inhibitor; beta-amyloid modulator | Antioxidant agent; NMDA receptor; antagonist; neuroprotectant |
| Dykellic acid [62,63] | Korea Research Institute of Bioscience and Biotechnology | Discovery | Immune disorder; ND; cancer | Apoptosis inhibitor; anticancer; antiapoptotic |
| IAP [64] | Aegera Therapeutics, Inc. | Discovery | PD; multiple sclerosis, cerebrovascular ischemia | Antiapoptotic; neuroprotectant |
| IDN-6556 [65,66] | Idun Pharmaceuticals, Inc. | Discovery | ND | Apoptosis inhibitor; anti-inflammatory |
| Rasagiline [67,68] | Teva Pharmaceutical Industries, Ltd. | Phase 3 clinical | AD; ND; PD | Apoptosis inhibitor; MAO B inhibitor; antiparkinsonian |
| SPM-914 [42,69] | Alviva Biopharmaceuticals, Inc./Schwarz | Discovery | PD; HD; motor neuron disease | Neurodegenerative disease; ParkinsonŌĆÖs disease; apoptosis inhibitor |
REFERENCES
1. Khan ZA. Photo period and ovarian physiology a chronobiological approach in zebra fish (Danio rerio) [dissertation] Gauhati University. 2017.
2. Khan ZA, Labala RK, Yumnamcha T, Devi SD, Mondal G, Sanjita Devi H, et al. Artificial Light at Night (ALAN), an alarm to ovarian physiology: a study of possible chronodisruption on zebrafish (Danio rerio). Sci Total Environ 2018;628-629:1407ŌĆō1421.


3. Khan ZA, Yumnamcha T, Rajiv C, Sanjita Devi H, Mondal G, Devi SD, et al. Melatonin biosynthesizing enzyme genes and clock genes in ovary and whole brain of zebrafish (Danio rerio): Differential expression and a possible interplay. Gen Comp Endocrinol 2016;233:16ŌĆō31.


4. Rajiv C, Sanjita Devi H, Mondal G, Dharmajyoti Devi S, Khan ZA, Yumnamcha T, et al. Cloning, phylogenetic analysis and tissue distribution of melatonin bio-synthesizing enzyme genes (Tph1, Aanat1, Aanat2 and Hiomt) in a tropical carp, Catla catla. Biol Rhythm Res 2017;48:371ŌĆō386.

5. Kim Y, Lee SJ, Park CS, Kim BJ, Lee CS, Cha B, et al. The mediating effect of eveningness on the indirect relationships between shorter sleep duration, inattention, depression with smartphone addiction tendency. Chronobiol Med 2020;2:32ŌĆō40.


6. Kripke DF. Delayed circadian rhythms and pars tuberalis dysfunction in mood disorders. Chronobiol Med 2020;2:1ŌĆō2.


9. Cho CH, Lee Y. The chronobiologic-based practical approach to shift work. Chronobiol Med 2019;1:103ŌĆō106.


10. Cajochen C, Kr├żuchi K, Wirz-Justice A. Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol 2003;15:432ŌĆō437.


11. Tarocco A, Caroccia N, Morciano G, Wieckowski MR, Ancora G, Garani G, et al. Melatonin as a master regulator of cell death and inflammation:molecular mechanisms and clinical implications for newborn care. Cell Death Dis 2019;10:317.



12. Binkley S, Mosher K, Rubin F, White B. Xenopus tadpole melanophores are controlled by dark and light and melatonin without influence of time of day. J Pineal Res 1988;5:87ŌĆō97.


15. Falc├│n J, Besseau L, Fuent├©s M, Sauzet S, Magnanou E, Boeuf G. Structural and functional evolution of the pineal melatonin system in vertebrates. Ann N Y Acad Sci 2009;1163:101ŌĆō111.


16. Falc├│n J, Besseau L, Sauzet S, Boeuf G. Melatonin effects on the hypothalamo-pituitary axis in fish. Trends Endocrinol Metab 2007;18:81ŌĆō88.


17. Falc├│n J, Migaud H, Mu├▒oz-Cueto JA, Carrillo M. Current knowledge on the melatonin system in teleost fish. Gen Comp Endocrinol 2010;165:469ŌĆō482.


18. Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci 2001;4:1165.



19. Zhao D, Yu Y, Shen Y, Liu Q, Zhao Z, Sharma R, et al. Melatonin synthesis and function: evolutionary history in animals and plants. Front Endocrinol (Lausanne) 2019;10:249.



20. Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol 2011;17:3888ŌĆō3898.



21. Gonzalez-Arto M, Hamilton TR, Gallego M, Gaspar-Torrubia E, Aguilar D, Serrano-Blesa E, et al. Evidence of melatonin synthesis in the ram reproductive tract. Andrology 2016;4:163ŌĆō171.


22. Scher J, Wankiewicz E, Brown GM, Fujieda H. MT(1) melatonin receptor in the human retina: expression and localization. Invest Ophthalmol Vis Sci 2002;43:889ŌĆō897.

23. Brennan R, Jan JE, Lyons CJ. Light, dark, and melatonin: emerging evidence for the importance of melatonin in ocular physiology. Eye (Lond) 2007;21:901ŌĆō908.



24. Jockers R, Delagrange P, Dubocovich ML, Markus RP, Renault N, Tosini G, et al. Update on melatonin receptors: IUPHAR Review 20. Br J Pharmacol 2016;173:2702ŌĆō2725.



25. Liu J, Clough SJ, Hutchinson AJ, Adamah-Biassi EB, Popovska-Gorevski M, Dubocovich ML. MT1 and MT2 melatonin receptors: a therapeutic perspective. Annu Rev Pharmacol Toxicol 2016;56:361ŌĆō383.


26. Ekmekcioglu C. Melatonin receptors in humans: biological role and clinical relevance. Biomed Pharmacother 2006;60:97ŌĆō108.


27. Reiter RJ, Tan DX, Manchester LC, Pilar Terron M, Flores LJ, Koppisepi S. Medical implications of melatonin: receptor-mediated and receptor-independent actions. Adv Med Sci 2007;52:11ŌĆō28.
28. Wang H, Pu Y, Luo L, Li Y, Zhang Y, Cao Z. Membrane receptor-independent inhibitory effect of melatonin on androgen production in porcine theca cells. Theriogenology 2018;118:63ŌĆō71.


29. Chen D, Mei Y, Kim N, Lan G, Gan CL, Fan F, et al. Melatonin directly binds and inhibits death-associated protein kinase 1 function in AlzheimerŌĆÖs disease. J Pineal Res 2020;69:e12665.




30. Koh PO. Melatonin prevents ischemic brain injury through activation of the mTOR/p70S6 kinase signaling pathway. Neurosci Lett 2008;444:74ŌĆō78.


32. Iwasaki S, Nakazawa K, Sakai J, Kometani K, Iwashita M, Yoshimura Y, et al. Melatonin as a local regulator of human placental function. J Pineal Res 2005;39:261ŌĆō265.


33. Nakazawa K, Kanakura Y, Kometani K, Iwasaki S, Yosimura Y. Study on melatonin in human and rat placental tissue. Placenta 1999;20 Suppl 1:467ŌĆō474.

35. Carloni S, Facchinetti F, Pelizzi N, Buonocore G, Balduini W. Melatonin acts in synergy with hypothermia to reduce oxygen-glucose deprivationinduced cell death in rat hippocampus organotypic slice cultures. Neonatology 2018;114:364ŌĆō371.


36. Motta-Teixeira LC, Machado-Nils AV, Battagello DS, Diniz GB, Andrade-Silva J, Silva S Jr, et al. The absence of maternal pineal melatonin rhythm during pregnancy and lactation impairs offspring physical growth, neurodevelopment, and behavior. Horm Behav 2018;105:146ŌĆō156.


37. Tan DX, Manchester LC, Reiter RJ, Qi WB, Zhang M, Weintraub ST, et al. Identification of highly elevated levels of melatonin in bone marrow: its origin and significance. Biochim Biophys Acta 1999;1472:206ŌĆō214.


38. Jeong S. Molecular and cellular basis of neurodegeneration in AlzheimerŌĆÖs disease. Mol Cells 2017;40:613ŌĆō620.



39. Tan SH, Karri V, Tay NWR, Chang KH, Ah HY, Ng PQ, et al. Emerging pathways to neurodegeneration: dissecting the critical molecular mechanisms in AlzheimerŌĆÖs disease, ParkinsonŌĆÖs disease. Biomed Pharmacother 2019;111:765ŌĆō777.


40. Callaway JK, Lawrence AJ, Jarrott B. AM-36, a novel neuroprotective agent, profoundly reduces reactive oxygen species formation and dopamine release in the striatum of conscious rats after endothelin-1-induced middle cerebral artery occlusion. Neuropharmacology 2003;44:787ŌĆō800.


42. Kwon MO, Fischer F, Matthisson M, Herrling P. List of drugs in development for neurodegenerative diseases. Neurodegener Dis 2004;1:113ŌĆō152.


43. Dhainaut A, Tizot A, Raimbaud E, Lockhart B, Lestage P, Goldstein S. Synthesis, structure, and neuroprotective properties of novel imidazolyl nitrones. J Med Chem 2000;43:2165ŌĆō2175.


44. Kelso GF, Porteous CM, Coulter CV, Hughes G, Porteous WK, Ledgerwood EC, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem 2001;276:4588ŌĆō4596.


45. Matsuoka Y, Jouroukhin Y, Gray AJ, Ma L, Hirata-Fukae C, Li HF, et al. A neuronal microtubule-interacting agent, NAPVSIPQ, reduces tau pathology and enhances cognitive function in a mouse model of AlzheimerŌĆÖs disease. J Pharmacol Exp Ther 2008;325:146ŌĆō153.


46. Pieper CM, Roza AM, Henderson JD Jr, Zhu YR, Lai CS. Spatial distribution and temporal onset of NF-kB activation and inducible nitric oxide synthase within pancreatic islets in the pre-diabetic stage of genetic, diabetic-prone BB rats: attenuation by drug intervention decreases inflammatory cell infiltration and incidence of diabetes. Inflamm Res 2004;53:22ŌĆō30.



47. Jiang ZG, Lebowitz MS, Ghanbari HA. Neuroprotective activity of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (PAN-811), a cancer therapeutic agent. CNS Drug Rev 2006;12:77ŌĆō90.



48. Poga─Źi─ć Kramp V, Herrling P. List of drugs in development for neurodegenerative diseases: update June 2010. Neurodegener Dis 2011;8:44ŌĆō94.


49. Valensi P, Le Devehat C, Richard JL, Farez C, Khodabandehlou T, Rosenbloom RA, et al. A multicenter, double-blind, safety study of QR-333 for the treatment of symptomatic diabetic peripheral neuropathy. A preliminary report. J Diabetes Complications 2005;19:247ŌĆō253.


50. Baek IS, Kim TK, Seo JS, Lee KW, Lee YA, Cho J, et al. AAD-2004 attenuates progressive neuronal loss in the brain of Tg-betaCTF99/B6 mouse model of Alzheimer disease. Exp Neurobiol 2013;22:31ŌĆō37.



52. Drummond NJ. Targeting a custom-engineered flavonoid to the mitochondria protects against acute oxidative stress [dissertation] The University of Edinburgh. 2015.
53. Golden TR, Patel M. Catalytic antioxidants and neurodegeneration. Antioxid Redox Signal 2009;11:555ŌĆō569.



54. Los M, Burek CJ, Stroh C, Benedyk K, Hug H, Mackiewicz A. Anticancer drugs of tomorrow: apoptotic pathways as targets for drug design. Drug Discov Today 2003;8:67ŌĆō77.


55. Murphy FJ, Seery LT, Hayes I. Therapeutic approaches to the modulation of apoptosis. Essays Biochem 2003;39:131ŌĆō153.



56. Roy J, Drapeau SJ, Marx JC, inventors; Warsaw Orthopedic Inc., assignee. Methods for treating back or neck pain caused by NGF using a therapeutic agent consisting of ReN-1820, ALE-0540 and capsaicin. United States patent US 9,789,161 B2. 2017 Oct 17.
57. Lombard DB, Kohler W, Guo AH, Gendron C, Han M, Ding W, et al. High throughput small molecule screening reveals NRF2-dependent and -independent pathways of cellular stress resistance. bioRxiv 2019;778548 [preprint]. Available from https://doi.org/10.1101/778548.

58. Lee JY, Friedman JE, Angel I, Kozak A, Koh JY. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol Aging 2004;25:1315ŌĆō1321.


59. Diener HC, Schneider D, Lampl Y, Bornstein NM, Kozak A, Rosenberg G. DP-b99, a membrane-activated metal ion chelator, as neuroprotective therapy in ischemic stroke. Stroke 2008;39:1774ŌĆō1778.


60. Khare E, Fatima Z. Recent advances and current perspectives in treatment of AlzheimerŌĆÖs disease. Environ Conserv J 2020;21:183ŌĆō186.

61. Nguyen TT, Giau VV, Vo TK. Current advances in transdermal delivery of drugs for AlzheimerŌĆÖs disease. Indian J Pharmacol 2017;49:145ŌĆō154.



62. Woo JH, Park JW, Lee SH, Kim YH, Lee IK, Gabrielson E, et al. Dykellic acid inhibits phorbol myristate acetate-induced matrix metalloproteinase-9 expression by inhibiting nuclear factor kappa B transcriptional activity. Cancer Res 2003;63:3430ŌĆō3434.

63. Heo JC, Park JY, Woo SU, Rho JR, Lee HJ, Kim SU, et al. Dykellic acid inhibits cell migration and tube formation by RhoA-GTP expression. Biol Pharm Bull 2006;29:2256ŌĆō2259.


64. Laurent A, Hewitt K, Morris S, Bureau P, Boudreault A, Jarvis S, et al., inventors;Pharmascience Inc., assignee. IAP BIR domain binding compounds. United States patent US 9,284,350. 2016 Mar 15.
66. Lee H, Shin EA, Lee JH, Ahn D, Kim CG, Kim JH, et al. Caspase inhibitors:a review of recently patented compounds (2013-2015). Expert Opin Ther Pat 2018;28:47ŌĆō59.


67. Statland JM, Moore D, Wang Y, Walsh M, Mozaffar T, Elman L, et al. Rasagiline for amyotrophic lateral sclerosis: a randomized, controlled trial. Muscle Nerve 2019;59:201ŌĆō207.


68. Weintraub D, Hauser RA, Elm JJ, Pagan F, Davis MD, Choudhry A; MODERATO Investigators. Rasagiline for mild cognitive impairment in ParkinsonŌĆÖs disease: a placebo-controlled trial. Mov Disord 2016;31:709ŌĆō714.


69.
Staunton J
Jin X
Rufo DS
Monteiro M
. Compositions and methods for the treatment of neurodegenerative diseases. World patent WO 2006/119329 A2. 2006 Nov 9.
70. Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol 2002;31:581ŌĆō593.


71. Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after ŌĆ£temporaryŌĆØ noise-induced hearing loss. J Neurosci 2009;29:14077ŌĆō14085.



72. Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation 2011;8:110.



73. Navarro X, Viv├│ M, Valero-Cabr├® A. Neural plasticity after peripheral nerve injury and regeneration. Prog Neurobiol 2007;82:163ŌĆō201.


74. Resende R, Moreira PI, Proen├¦a T, Deshpande A, Busciglio J, Pereira C, et al. Brain oxidative stress in a triple-transgenic mouse model of Alzheimer disease. Free Radic Biol Med 2008;44:2051ŌĆō2057.


75. Nishida Y, Yokota T, Takahashi T, Uchihara T, Jishage K, Mizusawa H. Deletion of vitamin E enhances phenotype of Alzheimer disease model mouse. Biochem Biophys Res Commun 2006;350:530ŌĆō536.


76. Li F, Calingasan NY, Yu F, Mauck WM, Toidze M, Almeida CG, et al. Increased plaque burden in brains of APP mutant MnSOD heterozygous knockout mice. J Neurochem 2004;89:1308ŌĆō1312.


77. Matsuoka Y, Picciano M, La Francois J, Duff K. Fibrillar beta-amyloid evokes oxidative damage in a transgenic mouse model of AlzheimerŌĆÖs disease. Neuroscience 2001;104:609ŌĆō613.


78. Chinopoulos C, Adam-Vizi V. Calcium, mitochondria and oxidative stress in neuronal pathology. Novel aspects of an enduring theme. FEBS J 2006;273:433ŌĆō450.


79. Schipper HM. Redox neurology: visions of an emerging subspecialty. Ann N Y Acad Sci 2004;1012:342ŌĆō355.


80. Li J, Wang C, Zhang JH, Cai JM, Cao YP, Sun XJ. Hydrogen-rich saline improves memory function in a rat model of amyloid-beta-induced AlzheimerŌĆÖs disease by reduction of oxidative stress. Brain Res 2010;1328:152ŌĆō161.


81. Padurariu M, Ciobica A, Hritcu L, Stoica B, Bild W, Stefanescu C. Changes of some oxidative stress markers in the serum of patients with mild cognitive impairment and AlzheimerŌĆÖs disease. Neurosci Lett 2010;469:6ŌĆō10.


82. Senoglu M, Nacitarhan V, Kurutas EB, Senoglu N, Altun I, Atli Y, et al. Intraperitoneal alpha-lipoic acid to prevent neural damage after crush injury to the rat sciatic nerve. J Brachial Plex Peripher Nerve Inj 2009;4:22.




83. Varija D, Kumar KP, Reddy KP, Reddy VK. Prolonged constriction of sciatic nerve affecting oxidative stressors & antioxidant enzymes in rat. Indian J Med Res 2009;129:587ŌĆō592.

84. Lee CL, Wang JJ, Pan TM. Red mold rice extract represses amyloid beta peptide-induced neurotoxicity via potent synergism of anti-inflammatory and antioxidative effect. Appl Microbiol Biotechnol 2008;79:829ŌĆō841.



85. Jang MH, Jung SB, Lee MH, Kim CJ, Oh YT, Kang I, et al. Melatonin attenuates amyloid beta25-35-induced apoptosis in mouse microglial BV2 cells. Neurosci Lett 2005;380:26ŌĆō31.


86. Lee SY, Lee JW, Lee H, Yoo HS, Yun YP, Oh KW, et al. Inhibitory effect of green tea extract on beta-amyloid-induced PC12 cell death by inhibition of the activation of NF-kappaB and ERK/p38 MAP kinase pathway through antioxidant mechanisms. Brain Res Mol Brain Res 2005;40:45ŌĆō54.

87. Hashimoto M, Tanabe Y, Fujii Y, Kikuta T, Shibata H, Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J Nutr 2005;135:549ŌĆō555.



88. Olcese JM, Cao C, Mori T, Mamcarz MB, Maxwell A, Runfeldt MJ, et al. Protection against cognitive deficits and markers of neurodegeneration by long-term oral administration of melatonin in a transgenic model of Alzheimer disease. J Pineal Res 2009;47:82ŌĆō96.


89. Ionov M, Burchell V, Klajnert B, Bryszewska M, Abramov AY. Mechanism of neuroprotection of melatonin against beta-amyloid neurotoxicity. Neuroscience 2011;180:229ŌĆō237.


90. Rosales-Corral S, Tan DX, Reiter RJ, Valdivia-Vel├Īzquez M, Mart├Łnez-Barboza G, Acosta-Mart├Łnez JP, et al. Orally administered melatonin reduces oxidative stress and proinflammatory cytokines induced by amyloid-beta peptide in rat brain: a comparative, in vivo study versus vitamin C and E. J Pineal Res 2003;35:80ŌĆō84.


91. Jesudason EP, Baben B, Ashok BS, Masilamoni JG, Kirubagaran R, Jebaraj WC, et al. Anti-inflammatory effect of melatonin on A beta vaccination in mice. Mol Cell Biochem 2007;298:69ŌĆō81.



92. Shen YX, Xu SY, Wei W, Wang XL, Wang H, Sun X. Melatonin blocks rat hippocampal neuronal apoptosis induced by amyloid beta-peptide 25-35. J Pineal Res 2002;32:163ŌĆō167.


93. Feng Z, Qin C, Chang Y, Zhang JT. Early melatonin supplementation alleviates oxidative stress in a transgenic mouse model of AlzheimerŌĆÖs disease. Free Radic Biol Med 2006;40:101ŌĆō109.


94. Olivieri G, Otten U, Meier F, Baysang G, Dimitriades-Schmutz B, M├╝ller-Spahn F, et al. Beta-amyloid modulates tyrosine kinase B receptor expression in SHSY5Y neuroblastoma cells: influence of the antioxidant melatonin. Neuroscience 2003;120:659ŌĆō665.


95. Anderson KN, Jamieson S, Graham AJ, Shneerson JM. REM sleep behaviour disorder treated with melatonin in a patient with AlzheimerŌĆÖs disease. Clin Neurol Neurosurg 2008;110:492ŌĆō495.


96. Shokouhi G, Tubbs RS, Shoja MM, Hadidchi S, Ghorbanihaghjo A, Roshangar L, et al. Neuroprotective effects of high-dose vs low-dose melatonin after blunt sciatic nerve injury. Childs Nerv Syst 2008;24:111ŌĆō117.



97. Rog├®rio F, Teixeira SA, de Rezende AC, de S├Ī RC, de Souza Queiroz L, De Nucci G, et al. Superoxide dismutase isoforms 1 and 2 in lumbar spinal cord of neonatal rats after sciatic nerve transection and melatonin treatment. Brain Res Dev Brain Res 2005;154:217ŌĆō225.


98. Stavisky RC, Britt JM, Zuzek A, Truong E, Bittner GD. Melatonin enhances the in vitro and in vivo repair of severed rat sciatic axons. Neurosci Lett 2005;376:98ŌĆō101.


99. Turgut M, Uyanikgil Y, Baka M, Tun├¦ AT, Yava┼¤o─¤lu A, Yurtseven ME, et al. Pinealectomy exaggerates and melatonin treatment suppresses neuroma formation of transected sciatic nerve in rats: gross morphological, histological and stereological analysis. J Pineal Res 2005;38:284ŌĆō291.


100. Zencirci SG, Bilgin MD, Yaraneri H. Electrophysiological and theoretical analysis of melatonin in peripheral nerve crush injury. J Neurosci Methods 2010;191:277ŌĆō282.


101. Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ 2006;13:385ŌĆō392.



102. Rayavarapu S, Coley W, Nagaraju K. Endoplasmic reticulum stress in skeletal muscle homeostasis and disease. Curr Rheumatol Rep 2012;14:238ŌĆō243.




103. Unterberger U, H├Čftberger R, Gelpi E, Flicker H, Budka H, Voigtl├żnder T. Endoplasmic reticulum stress features are prominent in Alzheimer disease but not in prion diseases in vivo. J Neuropathol Exp Neurol 2006;65:348ŌĆō357.



104. Lee JH, Won SM, Suh J, Son SJ, Moon GJ, Park UJ, et al. Induction of the unfolded protein response and cell death pathway in AlzheimerŌĆÖs disease, but not in aged Tg2576 mice. Exp Mol Med 2010;42:386ŌĆō394.



105. Hoozemans JJ, van Haastert ES, Nijholt DA, Rozemuller AJ, Eikelenboom P, Scheper W. The unfolded protein response is activated in pretangle neurons in AlzheimerŌĆÖs disease hippocampus. Am J Pathol 2009;174:1241ŌĆō1251.



106. Slodzinski H, Moran LB, Michael GJ, Wang B, Novoselov S, Cheetham ME, et al. Homocysteine-induced endoplasmic reticulum protein (herp) is up-regulated in parkinsonian substantia nigra and present in the core of Lewy bodies. Clin Neuropathol 2009;28:333ŌĆō343.

107. Carnemolla A, Fossale E, Agostoni E, Michelazzi S, Calligaris R, De Maso L, et al. Rrs1 is involved in endoplasmic reticulum stress response in Huntington disease. J Biol Chem 2009;284:18167ŌĆō18173.



108. Ling ZQ, Tian Q, Wang L, Fu ZQ, Wang XC, Wang Q, et al. Constant illumination induces Alzheimer-like damages with endoplasmic reticulum involvement and the protection of melatonin. J Alzheimers Dis 2009;16:287ŌĆō300.


109. Lin AM, Fang SF, Chao PL, Yang CH. Melatonin attenuates arsenite-induced apoptosis in rat brain: involvement of mitochondrial and endoplasmic reticulum pathways and aggregation of alpha-synuclein. J Pineal Res 2007;43:163ŌĆō171.


110. Kang EB, Kwon IS, Koo JH, Kim EJ, Kim CH, Lee J, et al. Treadmill exercise represses neuronal cell death and inflammation during A╬▓-induced ER stress by regulating unfolded protein response in aged presenilin 2 mutant mice. Apoptosis 2013;18:1332ŌĆō1347.



111. Lin YW, Chen TY, Hung CY, Tai SH, Huang SY, Chang CC, et al. Melatonin protects brain against ischemia/reperfusion injury by attenuating endoplasmic reticulum stress. Int J Mol Med 2018;42:182ŌĆō192.



113. L├│pez A, Garc├Ła JA, Escames G, Venegas C, Ortiz F, L├│pez LC, et al. Melatonin protects the mitochondria from oxidative damage reducing oxygen consumption, membrane potential, and superoxide anion production. J Pineal Res 2009;46:188ŌĆō198.


114. Escames G, L├│pez LC, Ortiz F, L├│pez A, Garc├Ła JA, Ros E, et al. Attenuation of cardiac mitochondrial dysfunction by melatonin in septic mice. FEBS J 2007;274:2135ŌĆō2147.


115. Rodr├Łguez MI, Carretero M, Escames G, L├│pez LC, Maldonado MD, Tan DX, et al. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic Res 2007;41:15ŌĆō24.


116. Hardeland R, Cardinali DP, Srinivasan V, Spence DW, Brown GM, Pandi-Perumal SR. Melatonin--a pleiotropic, orchestrating regulator molecule. Prog Neurobiol 2011;93:350ŌĆō384.


117. Hardeland R. Neuroprotection by radical avoidance: search for suitable agents. Molecules 2009;14:5054ŌĆō5102.



118. Dungel P, Mittermayr R, Haindl S, Osipov A, Wagner C, Redl H, et al. Illumination with blue light reactivates respiratory activity of mitochondria inhibited by nitric oxide, but not by glycerol trinitrate. Arch Biochem Biophys 2008;471:109ŌĆō115.


119. Hardeland R, Poeggeler B, Niebergall R, Zelosko V. Oxidation of melatonin by carbonate radicals and chemiluminescence emitted during pyrrole ring cleavage. J Pineal Res 2003;34:17ŌĆō25.


120. Hardeland R. Antioxidative protection by melatonin: multiplicity of mechanisms from radical detoxification to radical avoidance. Endocrine 2005;27:119ŌĆō130.


121. Reiter RJ, Tan DX, Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech Ageing Dev 2002;123:1007ŌĆō1019.


122. Reiter RJ. Interactions of the pineal hormone melatonin with oxygen-centered free radicals: a brief review. Braz J Med Biol Res 1993;26:1141ŌĆō1155.

123. Yon JH, Daniel-Johnson J, Carter LB, Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 2005;135:815ŌĆō827.


124. Henry-Mowatt J, Dive C, Martinou JC, James D. Role of mitochondrial membrane permeabilization in apoptosis and cancer. Oncogene 2004;23:2850ŌĆō2860.



125. Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie 2002;84:153ŌĆō166.


126. Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Jou MJ, Acuna-Castroviejo D. Melatonin mitigates mitochondrial meltdown: interactions with SIRT3. Int J Mol Sci 2018;19:2439.



127. Mart├Łn M, Mac├Łas M, Escames G, Le├│n J, Acu├▒a-Castroviejo D. Melatonin but not vitamins C and E maintains glutathione homeostasis in t-butyl hydroperoxide-induced mitochondrial oxidative stress. FASEB J 2000;14:1677ŌĆō1679.


128. Le├│n J, Acu├▒a-Castroviejo D, Escames G, Tan DX, Reiter RJ. Melatonin mitigates mitochondrial malfunction. J Pineal Res 2005;38:1ŌĆō9.


129. Mart├Łn M, Mac├Łas M, Le├│n J, Escames G, Khaldy H, Acu├▒a-Castroviejo D. Melatonin increases the activity of the oxidative phosphorylation enzymes and the production of ATP in rat brain and liver mitochondria. Int J Biochem Cell Biol 2002;34:348ŌĆō357.


130. Acuna-Castroviejo D, Escames G, Rodriguez MI, Lopez LC. Melatonin role in the mitochondrial function. Front Biosci 2007;12:947ŌĆō963.


131. Kilic E, Kilic U, Yulug B, Hermann DM, Reiter RJ. Melatonin reduces disseminate neuronal death after mild focal ischemia in mice via inhibition of caspase-3 and is suitable as an add-on treatment to tissue-plasminogen activator. J Pineal Res 2004;36:171ŌĆō176.


132. Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 1997;275:1129ŌĆō1132.


133. Jou MJ, Peng TI, Reiter RJ, Jou SB, Wu HY, Wen ST. Visualization of the antioxidative effects of melatonin at the mitochondrial level during oxidative stress-induced apoptosis of rat brain astrocytes. J Pineal Res 2004;37:55ŌĆō70.


134. Kroemer G, Petit P, Zamzami N, Vayssi├©re J-L, Mignotte B. The biochemistry of programmed cell death. FASEB J 1995;9:1277ŌĆō1287.


135. Chovancova B, Hudecova S, Lencesova L, Babula P, Rezuchova I, Penesova A, et al. Melatonin-induced changes in cytosolic calcium might be responsible for apoptosis induction in tumour cells. Cell Physiol Biochem 2017;44:763ŌĆō777.


136. Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 2012;45:487ŌĆō498.



137. Miura M. Apoptotic and non-apoptotic caspase functions in neural development. Neurochem Res 2011;36:1253ŌĆō1260.



138. Ghavami S, Shojaei S, Yeganeh B, Ande SR, Jangamreddy JR, Mehrpour M, et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog Neurobiol 2014;112:24ŌĆō49.


139. Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, et al. Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 1998;94:739ŌĆō750.


140. Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P. Apaf1 (CED-4 homolog) regulates programmed cell death in mammalian development. Cell 1998;94:727ŌĆō737.


141. Motoyama N, Wang F, Roth KA, Sawa H, Nakayama K, Nakayama K, et al. Massive cell death of immature hematopoietic cells and neurons in Bclx-deficient mice. Science 1995;267:1506ŌĆō1510.


142. Naseem M, Parvez S. Role of melatonin in traumatic brain injury and spinal cord injury. ScientificWorldJournal 2014;2014:586270.




143. Khalatbary AR, Ahmadvand H. Anti-inflammatory effect of the epigallocatechin gallate following spinal cord trauma in rat. Iran Biomed J 2011;15:31ŌĆō37.


144. Cheung RT. The utility of melatonin in reducing cerebral damage resulting from ischemia and reperfusion. J Pineal Res 2003;34:153ŌĆō160.


145. Baydas G, Reiter RJ, Akbulut M, Tuzcu M, Tamer S. Melatonin inhibits neural apoptosis induced by homocysteine in hippocampus of rats via inhibition of cytochrome c translocation and caspase-3 activation and by regulating pro- and anti-apoptotic protein levels. Neuroscience 2005;135:879ŌĆō886.


146. Koh PO. Melatonin attenuates the focal cerebral ischemic injury by inhibiting the dissociation of pBad from 14-3-3. J Pineal Res 2008;44:101ŌĆō106.


147. Shi L, Liang F, Zheng J, Zhou K, Chen S, Yu J, et al. Melatonin regulates apoptosis and autophagy via ROS-MST1 pathway in subarachnoid hemorrhage. Front Mol Neurosci 2018;11:93.



148. Das G, Shravage BV, Baehrecke EH. Regulation and function of autophagy during cell survival and cell death. Cold Spring Harb Perspect Biol 2012;4:a008813.



149. Carloni S, Girelli S, Scopa C, Buonocore G, Longini M, Balduini W. Activation of autophagy and Akt/CREB signaling play an equivalent role in the neuroprotective effect of rapamycin in neonatal hypoxia-ischemia. Autophagy 2010;6:366ŌĆō377.


150. Puyal J, Vaslin A, Mottier V, Clarke PG. Postischemic treatment of neonatal cerebral ischemia should target autophagy. Ann Neurol 2009;66:378ŌĆō389.


151. Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, et al. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 2008;4:762ŌĆō769.


152. Park S, Lee SK, Park K, Lee Y, Hong Y, Lee S, et al. Beneficial effects of endogenous and exogenous melatonin on neural reconstruction and functional recovery in an animal model of spinal cord injury. J Pineal Res 2012;52:107ŌĆō119.


153. Guo Y, Wang J, Wang Z, Yang Y, Wang X, Duan Q. Melatonin protects N2a against ischemia/reperfusion injury through autophagy enhancement. J Huazhong Univ Sci Technolog Med Sci 2010;30:1ŌĆō7.



154. Zheng Y, Hou J, Liu J, Yao M, Li L, Zhang B, et al. Inhibition of autophagy contributes to melatonin-mediated neuroprotection against transient focal cerebral ischemia in rats. J Pharmacol Sci 2014;124:354ŌĆō364.


155. Tang P, Hou H, Zhang L, Lan X, Mao Z, Liu D, et al. Autophagy reduces neuronal damage and promotes locomotor recovery via inhibition of apoptosis after spinal cord injury in rats. Mol Neurobiol 2014;49:276ŌĆō287.



156. Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006;10:51ŌĆō64.