Melatonin: A Potent Therapeutic Candidate in Degenerative Neural Damages
Article information
Abstract
The hormone of darkness “melatonin” has shown extraordinary potential. Owing to versatile properties which include, but are not limited to, strong antioxidation, anti-inflammatory, anti-apoptotic, and chronotherapeutic action has generated a great deal of interest as a therapeutic drug for various diseases especially neural damage and neurodegenerative diseases. Melatonin has shown to be effective in arresting neurodegeneration in experimental models of Alzheimer’s, Huntington’s, and Parkinson’s disease, subarachnoid hemorrhage, and ischemic stroke. In this article, the authors review the role of melatonin in neural damage and associated diseases. Briefly, we will discuss the function and protective role of melatonin in the central and peripheral nervous systems. Subsequently, an overview of the role of melatonin in endoplasmic reticulum and mitochondria in neural tissues along with possible biological pathways will be addressed. Finally, we will review the action of melatonin on programmed cell death and its probable molecular mechanism.
INTRODUCTION
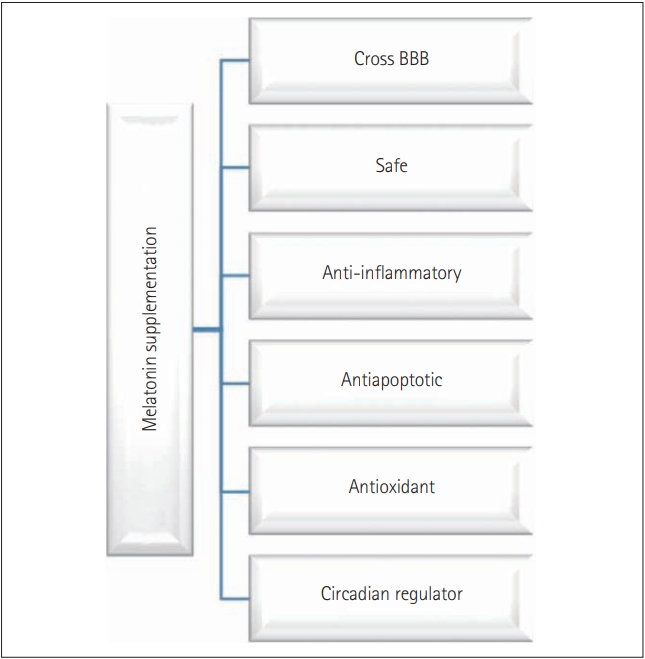
Melatonin (N-acetyl-5-methoxytryptamine), commonly known as the sleep hormone or hormone of darkness, is a phylogenetically conserved molecule with numerous versatile and diverse functions [1-4]. In general, melatonin has; 1) antioxidant activity primarily by its free radical scavenging, 2) antiapoptotic property mostly by blocking caspase-3 cleavage and mitochondrial permeability transition pore opening, 3) anti-inflammatory action generally by inhibiting inflammasome activation, and 4) chronobiotic abilities by acting as a sleep inducer [5-11]. Moreover, in different organisms, the function of melatonin includes maintenance of gut motility, circadian and sedation (sleep) activity, immune modulation, osmoregulation, shoaling and schooling, locomotor activity, regulation of cell cycle, food intake, thermal preference management, skin pigmentation, reproduction and growth [12-17]. This multifunctional and multipotent indolamine is primarily produced in the pineal gland in a circadian manner synchronized with photoperiodic information received via the retinohypothalamic pathway [18]. Recent studies have proved that melatonin can be synthesized from several extra pineal sites like retina, brain, gut, testis, and ovary [4,19-22]. The presence of melatonin receptors in almost all of the vertebrate tissues is reported, which is an indicator of the functional prowess of melatonin [22-26]. Melatonin is not a conventional hormone as it can functions via receptor-dependent and receptor-independent manner by binding with nuclear binding sites or serine/threonine (Ser/Thr) kinases like death-associated protein kinase 1 [27-30]. Additionally, melatonin is amphiphilic, this character of melatonin provides it the ability to diffuse and cross all morpho-physiological barriers, enter all the cells, and influence numerous function [31-37]. Owing to the virtual omnipresence, cytoprotective, and chronobiotic abilities along with biocompatibility, melatonin is considered as a powerful therapeutic drug for the treatment of various diseases (Figure 1). As melatonin can cross-blood brain barrier and enter any cell and modulate many functions, melatonin is used as a drug for many degenerative neural damages such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD) or amyotrophic lateral sclerosis, etc.
Progressive degradation of the neurons in the sensory, motor, or cognitive system is the primary characteristic of neurodegenerative disorders leading to diseases such as AD, dementia, PD, or HD. Neurodegeneration in all these disorders follows similar molecular processes like oxidation mediated degeneration, impairment of mitochondria, excitotoxicity, and inflammation [38,39]. Even though the regular intake of drugs with antioxidants, antiinflammatory, and antiapoptotic activity has been proposed for management or treatment of neural damage, however, their effectiveness has been questioned and most of them are under the primary stages of drug development (Table 1) [40-69]. In this context, melatonin simultaneously offers antioxidant, anti-inflammatory, and antiapoptotic activity. Therefore, melatonin is considered as a promising target for the treatment or management of neurodegenerative diseases. The focus of this article is on the role of melatonin in neural tissues. We will discuss the role of melatonin in the central nervous system (CNS) and peripheral nervous system (PNS). Following that, an overview of functions of melatonin in the endoplasmic reticulum (ER) and mitochondria in neural tissues will be addressed. Finally, we will review the role of melatonin on apoptotic cell death and autophagy and its associated pathway.
THE EFFECTS OF MELATONIN ON THE CENTRAL AND PERIPHERAL NERVOUS SYSTEM
Neural tissues, located in both the CNS and PNS, exhibit degenerative changes with age. Even though the formation of new myelin has been reported to occur, myelin sheaths with dense cytoplasm and balloons have also been observed [70]. As these occurrences affect functions such as conduction velocity and normal timing in the neural circuit, structural changes of myelin lead to cognitive decline with age [70]. Furthermore, neural degeneration in the PNS is induced by detrimental stimuli or injury [71,72]. Peripheral nerve injury typically results in the loss of sensory, motor, and autonomic functions either partially or totally. Damage induced by peripheral nerve injury occurs from the original lesioned nerves to the denervated segments of the body due to the discontinuity of axons, degeneration of nerve fibers, and eventual death of injured neurons [73]. Previous studies have found increased oxidative stress in the neural tissues of subjects from an AD animal model [74-77]. Reactive oxygen species (ROS) are primarily generated by the Fenton reaction, which induces intracellular mitogen-activated protein kinase (MAPK) activation, Ca2+ dyshomeostasis, and apoptosis [78,79]. Interestingly, the oxidative condition is correlated with the clinical symptoms of AD such as memory deficits and cognitive impairments in both rodents and humans [80,81]. Similarly, sciatic nerve crush injury is a well-established rodent model due to its ability to impair axonal connectivity [82]. There is an up-regulation of oxidative stress-induced lipid peroxidation as well as a down-regulation of glutathione (GSH) and catalase in the sciatic nerve constriction model [83]. Cumulatively, ROS generation is induced when nerves are injured in both CNS and PNS. Therefore, researchers have focused on potent antioxidant substances that inhibit the progression of AD. Numerous studies have demonstrated the antioxidant effects of natural products or chemicals in vitro and in vivo and have shown that decreased oxidative stress improves neurological function in rodents with Aβ-induced AD and humans [80,84-87]. Olcese et al. [88] observed a beneficial effect of melatonin regarding the prevention of cognitive deficits and the suppression of oxidative stress-induced neurodegeneration via the up-regulation of antioxidant enzymes [e.g., superoxide dismutase (SOD-1), glutathione peroxidase, and catalase] and enhancement of the anti-inflammatory response by down-regulation of tumor necrosis factor-alpha (TNFα). Recently, the regulatory functions of melatonin, which are involved in its neuroprotective action against Aβ-induced neurotoxicity, were identified. It was shown that the neuroprotective effects under Aβ-induced toxicity are mediated by the ROS scavenging and the protection of astrocytes from mitochondrial depolarization [89]. In addition to its antioxidant properties, melatonin produces anti-inflammatory and antiapoptotic activity and increases neurotrophin production during AD progression both in vitro and in vivo [90-94]. These studies performed with AD patients have found a significant association between melatonin administration and improved sleep patterns [95]. Therefore, it may be beneficial to focus not only on the role of melatonin during primary neuropathological progression but also on secondary AD-induced problems such as the interruption of sleep. Shokouhi et al. [96] found that melatonin reduces lipid peroxidation in sciatic nerve-injured animals in a dose-dependent manner. Also, in the sciatic nerve transection model, treatment with melatonin reduces motor neuron loss in the lumbar spinal cord, stimulates SOD1/2 production, and aids in the recovery of action potentials in the crushed sciatic nerve both in vitro and in vivo [97,98]. Melatonin also inhibits collagen deposition in the neuroma formed by the injured sciatic nerve, whereas melatonin depletion, as induced by pinealectomy, increases collagen content [99]. Moreover, compared to control, melatonin treatment elevates the sciatic functional index, improves conduction velocity, and attenuates latency values as measured by in vivo electromyography; with no difference between low (5 mg/kg) and high (20 mg/kg) doses [100]. Therefore, it can be concluded that melatonin can stimulate neural regeneration through a decrease of pro-oxidants and the up-regulation of neurotrophic factors.
THE EFFECTS OF MELATONIN ON ENDOPLASMIC RETICULUM IN NEURAL TISSUES
The ER has important functions, which include the synthesis, folding, and translational modification of proteins. ER stress occurs when ER homeostasis is abnormally regulated and results in the activation of unfolded protein responses (UPR) to reduce ER stress [101]. UPRs are initiated by three ER membrane-associated proteins, PRKR-like endoplasmic reticulum kinase (PERK), inositol requiring enzyme 1 (IRE), and activating transcription factor 6 (ATF6α), which up-regulate the expression of ER chaperones such as binding immunoglobulin protein/glucose-regulated protein 78 and 94 (Bip/Grp78 and Grp94) [102]. The accumulation of misfolded proteins, which activate UPRs, is a common feature of many neurodegenerative diseases, including AD, PD, and HD [103-107]. These studies indicate the importance of ER homeostasis and its role in the degenerative changes of neural tissues.
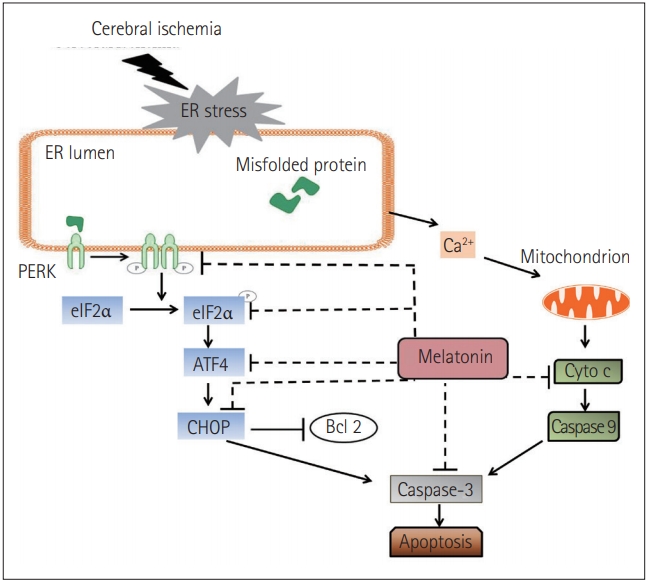
Interestingly, there are AD-like phenotypes, including spatial memory impairment, tau hyperphosphorylation, and the up-regulation of ER chaperones (e.g., BiP/GRP78, CHOP/GADD153), in illumination-induced melatonin-deprived rats [108]. Brain damage induced by Arsenite exposure increases ATF4, C/EBP homologous protein (CHOP), sXBP1, and pro-caspase 12 levels whereas, melatonin represses the expressions of these factors and leads to an inhibition of apoptosis [109]. In another report, Kang et al. [110] found that treadmill exercise suppresses ER stress responses via the inhibition of PERK, eukaryotic translation initiation factor 2α (eIF2α), ATF6α, sXBP1, and CHOP activation, reduces the deposition of Aβ-peptide42, and prevents memory dysfunction in aged PS2 mutant mice. Recently, it has been shown that in cerebral ischemia mice, melatonin operates with the same pathway. It not only decreases the phosphorylation of PERK and eIF2α but also suppresses the expression of ATF4 and CHOP. Simultaneously, the level of cytochrome c (Cyto c) was reduced and the expression of caspase-3 was down-regulated (Figure 2) [111]. Based on the aforementioned evidence, it can be concluded that melatonin contributes to the amelioration of neural degeneration through ER-associated signaling pathways.
THE EFFECT OF MELATONIN ON MITOCHONDRIA IN NEURAL TISSUES
A growing amount of evidence indicates that mitochondria play a pivotal role in the pathophysiology of various diseases [112]. Mitochondrial damage can occur through the impairment of electron flux via the electron transport chain, which results in electron leakage and generates oxidation in a self-stimulatory feedback loop. Recent studies have shown that melatonin can attenuate the generation of ROS and reactive nitrogen species (RNS) from mitochondria under several detrimental conditions and protect against the oxidative, nitrosative, and nitrative damage of electron transport chain proteins. Additionally, melatonin prevents lipid peroxidation in the inner membrane of mitochondria, which leads to electron flux and energy efficiency [113-115]. To understand the mechanisms underlying these findings, the site of radical generation in mitochondria and the causes of mitochondrial dysfunction should be addressed.
Melatonin has been shown to negatively modulate free radicals, and the regulation of inducible nitric oxide (NO) synthase (iNOS) and neuronal NO synthase (nNOS) [116]. Inflammatory and stressassociated signaling primarily up-regulates iNOS in astrocytes, microglia, and macrophages. In contrast, excitation dependent Ca2+ influx affects the regulation of nNOS, especially in glutamatergic neurons. Although a reasonable level of increase in NO radicals is likely to be suitable for mitochondrial function, significantly high levels often induce severe impairments in the electron transfer chain (ETC) [117]. The adverse effect of the NO radical and its non-enzymatically formed metabolites are responsible for the impairment of ETC, especially under detrimental conditions such as chronic inflammation [118]. Melatonin has been reported to have a potent influence on carbonate radicals and to be a scavenger of hydroxyl radicals [119-122]. Furthermore, the nitrosation of armates has been observed in the nitrosodioxyl radical, an analog of peroxynitrite that could be formed by electron abstraction from peroxynitrite; however, this has not been studied in electron transport chain proteins. If melatonin is administrated at high pharmacological doses, it might react with products mostly originating from these peroxynitrite. Nonetheless, at low pharmacological concentrations, the potential to inhibit the up-regulated iNOS or nNOS exists. This action of melatonin can break the malicious cycle of high NO radicals, which leads to a blockade of the electron transport chain in various ways, including by combining with the superoxide anion to produce strongly reactive factors that impair respirasomes, interrupt electron flux, and induce electron backflow and leakage.
Mitochondrion-dependent apoptosis, also termed the intrinsic apoptotic pathway, is one of the major apoptotic signaling pathways [123]. Mitochondrion-dependent apoptosis causes mitochondrial membrane permeabilization (MMP), caused by the opening of mitochondrial permeability transition pore (mPTP), which refers to the swelling and depolarization of mitochondria evident upon the development of a cytosolic calcium burst or when oxidative stress is in play [124-126]. MMP is thought to block ATP synthesis by oxidative phosphorylation in mitochondria and to stimulate ATP hydrolysis [125]. Melatonin levels have been reported to be higher inside the mitochondria than in the plasma [127]. Moreover, melatonin is thought to contribute to mitochondrial homeostasis [128,129] by 1) facilitating hydrogen peroxide (H2O2) scavenging (H2O2 is one of the most important ROS produced by mitochondria) and 2) improving mitochondrial function (increasing ATP synthesis, reducing ROS production, and protecting against abnormal decreases in mitochondrial membrane potential which could, in turn, trigger MMP) [130,131]. The release of Cyto c from mitochondria into the cytosol can initiate mitochondrion-dependent apoptosis via downstream activation of cell death pathways [132]. Melatonin not only blocks mPTP opening but also the mPTP-dependent release of Cyto c, whose release would inhibit caspase 3, triggering apoptotic cell death [133]. Some authors consider that ROS generation, caused by a decrease in mitochondrial membrane potential, may trigger mitochondrion-dependent apoptosis [128,134]. Similarly, the opening of mPTP under oxidative stress may play a role in the induction of apoptosis [128]. Melatonin maintains mitochondrial membrane permeability by preventing lipid peroxidation and also controls mitochondrial H2O2 levels, both under normal conditions and when oxidative stress is in play [11,128]. Additionally, melatonin seems to inhibit the ROS-induced increase in calcium levels [135]. Oxidative stress is a powerful regulator of the sensitivity of mPTPs to calcium ions. Thus, melatonin indirectly blocks the opening of the mPTPs and the induction of apoptotic cascades via the release of mitochondrial Cyto c and activation of caspase 3 [11].
Endogenous levels or exogenous administration of melatonin offers protection to injured cells from several cell death forms like, mPTP-driven cell death, necroptosis, apoptosis, and autophagy. Melatonin is highly permeable in a variety of cells organelles too, and its protective effects can be mediated through both melatonin receptor-dependent or independent manner. Inside the cytoplasm, melatonin inhibits the receptor-interacting serine/threonine-protein kinase 3 (Ripk3) mechanisms, up-regulation of dynamin-related protein (Drp1), and Bax-dependent Cyto c release caused by external insults, leading to pro-survival signals. Mitochondrial melatonin offers protective roles by interaction with peptide transporter 1 (PEPT1/2) and Glucose transporter (GLUT), which are considered as the novel melatonin receptors in the mitochondrion (Figure 3) [11].
THE EFFECTS OF MELATONIN ON PROGRAMMED CELL DEATH IN NEURAL TISSUES
Programmed cell death (PCD), refers to cell death mediated by intracellular programs. Three forms of PCD are known: apoptosis, autophagy, and programmed necrosis, apoptosis is most well understood [136]. PCD is important during neural development as it plays a central role in rendering the neuronal environment by regulating both the numbers and types of cells in the CNS [137,138]. Apoptosis is critical in regulating neuronal cell death, both during neural development and in various neurodegenerative diseases. Lower expression of apoptotic protease activating factor 1 (Apaf1) expression, can lead to abnormal brain enlargement because of apoptosis deregulation and may cause prenatal fatality in experimental animals [139,140]. Some studies have shown that excessive apoptotic cell death attributed to the lack of the antiapoptotic protein Bcl-x may cause neuronal immaturity in the developing brain and spinal cord leading to embryonic death [141]. Moreover, it is also shown that primary spinal cord injury is generally followed by apoptosis, inflammation, free-radical-induced lipid peroxidation, and vascular damage [142,143].
A considerable amount of literature has shown that apoptotic cell death can be affected by melatonin. Neural injuries elicit excitotoxicity, alteration of calcium ion homeostasis, increased nitric oxide synthesis, and finally, apoptosis [30,144]. Melatonin seems to protect neuronal cells from apoptotic cell death-mediated neurotoxicity by regulating the expression of proteins by enhancement of the interaction between pBad and 14-3-3, inhibiting activation of apoptosis cascades [145,146]. In subarachnoid hemorrhage (SAH), exogenous melatonin can reduce SAH-induced early brain injury by inhibiting excessive neuronal apoptosis and autophagy probably by the reactive oxygen species–mammalian sterile 20-like kinase 1 (ROS-MST1) pathway (Figure 4) [147].
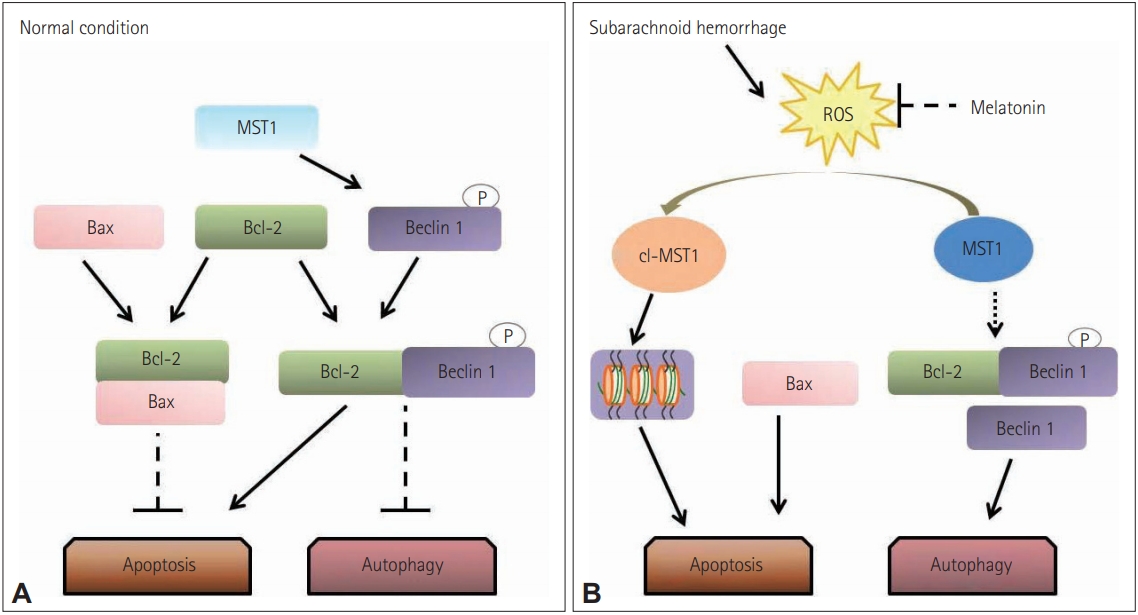
Proposed role of melatonin in regulating the balance between apoptosis and autophagy. (A) In normal situations, Bcl-2 binds with Bax and inhibiting the Bax-induced channel formation which is permeable to cytochrome c to activate caspase-3 dependent apoptosis. Additionally, MST1 present in the cell helps in cell survival by phosphorylating Beclin 1 which prevents the synthesis of Beclin 1-Atg14L-Vps34, which can inhibit autophagy and activate apoptosis. (B) In subarachnoid hemorrhage stress, the increased brain reactive oxygen species (ROS) content activates the cleavage of MST1 to produce cl-MST1, which is transferred into the nucleus and phosphorylates several histones, inducing neuronal cell apoptosis. Besides, subarachnoid hemorrhage reduces the level of Bcl-2 protein, displacing Bax from Bcl-2 to enhance apoptosis. In the meantime, both the down-regulation of Beclin 1 phosphorylation by MST1 and the low expression of Bcl-2 cause the dissociation of the Bcl-2/Beclin 1 complex, increasing the cell autophagy. Reprinted form Shi et al. Front Mol Neurosci 2018;11:93 [147], according to the Creative Commons License (CC-BY).
Autophagy, another type of PCD, is critical as it balances the formation and degradation of proteins, therefore, autophagy is not only a cell death pathway, but it can also promote cell survival [138,148]. Recently, autophagy has been considered as a pro-survival signaling pathway as it has shown a protective effect against necrotic cell death in neonatal rats subjected to brain damage by hypoxia-ischemia [149]. Conversely, deleterious aspects of autophagy have also been reported. After middle cerebral artery occlusion, the size of infarct was reduced upon injection of an inhibitor of autophagy [150]. Additionally, both brain edema and total infarct volume were also reduced by such an autophagy inhibitor [151]. After traumatic brain injury, autophagy is thought to induce cell death. Consequently, the effect of autophagy on the fate of cells remains ambiguous.
It has recently been shown that melatonin regulates autophagy. Park et al. [152] have shown melatonin induced rapid activation of autophagy after spinal cord injury. Inversely, Guo et al. [153] suggested that inhibition of autophagy could eliminate any beneficial role played by melatonin. The same study also reported the neuroprotective role of autophagy was augmented by melatonin, indicating that activation of autophagy might be protective [153]. To explain the Janus-faced nature of autophagy, it was suggested that melatonin-mediated autophagy induces autophagic cell death only if the extent of cellular damage exceeds a certain level, whereas it promotes cell survival if the damage is less extensive [153]. Conversely, other researchers have found that melatonin can be anti-autophagic. It has been proposed that melatonin plays a protective role after brain injury by activating the Class I Phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway, which negatively regulates autophagy [154]. Similarly, it has been suggested that melatonin exerts beneficial effects after brain injury by activating the rapamycin kinase pathway (mTOR/p70S6 kinase), the downstream target of Akt [30]. Activation of autophagy by inhibiting apoptosis can be beneficial in several circumstances. Such activation improves locomotor function in animals with spinal cord injuries [155]. A tumor-suppressing effect was also evident [74], as was the melatonin-induced improvement of cell survival in animal models of SAH [156,157]. Interestingly, the simultaneous down-regulation of apoptosis and autophagy may exert protective effects in animal models of brain injury [158]. Future studies should explore the details of the complex interactions between the mechanisms of PCD and neural injury.
SUMMARY AND CONCLUSION
Oxidative stress and inflammatory response leading to different modes of PCD have been involved in the pathogenesis of various neurodegenerative diseases such as AD, PD, HD and other neurological conditions including stroke, SAH, SCI, and brain trauma. Generally, high oxygen utilization and low anti-antioxidant levels are the main reasons for enhanced oxidative stress in damaged neural tissue and aged individuals. Melatonin is an excellent drug both in vivo and in vitro in neurodegenerative diseases. Melatonin has been shown to reduce derogatory neuropathological changes not only by ROS and RNS scavenging but also by decreasing the phosphorylation of PERK/eIF2α and suppressing ATF4/CHOP which down-regulates the caspase-3 mediated apoptosis. Furthermore, melatonin may also inhibit excessive neuronal apoptosis and autophagy, possibly by the ROS-MST1 pathway. The fact that melatonin easily crosses the blood-brain barrier, enters any cell, acts with and without the receptor, along with its high tolerance and biocompatibility even at the highest dosages, represents the significance of melatonin in treatment or management of neural damage. However, the number of randomized controlled studies testing the efficacy of melatonin on neural damage and neurodegenerative disorders is limited, and the experiment quality is often unsatisfactory. Lack of potential biomarkers to study the changes in neural damages after drug treatment makes these studies more challenging. There is a need for more clinical trials to study the protective role of melatonin. Moreover, the search for reliable biomarkers to study the post-drug administration changes in neural damage should be expedited.
Acknowledgements
The authors would like to acknowledge the invaluable support and critical comments of members in ‘Biological Clock & Aging Control’ laboratory. This work was supported by a grant from the National Research Foundation (NRF-2020R1A2C201215511), Korea. Jeonghyun Choi is supported by a post-doctoral fellowship from National Research Foundation (NRF-2019R1A6A3A01091422), Korea.
Notes
The authors have no potential conflicts of interest to disclose.
Author Contributions
Conceptualization: Yonggeun Hong, Yunkyung Hong, Zeeshan Ahmad Khan. Data curation: Zeeshan Ahmad Khan, Yunkyung Hong, Jeonghyun Choi, Youngjeon Lee, Yunho Jin. Project administration: Yonggeun Hong. Resources: Yonggeun Hong. Supervision: Yonggeun Hong. Writing—original draft: Zeeshan Ahmad Khan, Yunkyung Hong, Yonggeun Hong. Writing—review & editing: Yonggeun Hong, Zeeshan Ahmad Khan.